More Information
Submitted: March 28, 2023 | Approved: April 24, 2023 | Published: April 25, 2023
How to cite this article: Marinaccio M, Christopher C, Valeria P, Zaza C, Falcicchio G, et al. Single brain metastasis as onset of stage I endometrial carcinoma in patient affected by multiple sclerosis: the first case in literature. Arch Surg Clin Res. 2023; 7: 012-015.
DOI: 10.29328/journal.ascr.1001068
Copyright License: © Marinaccio M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Brain metastasis; Endometrial cancer; Multiple sclerosis
Single brain metastasis as onset of stage I endometrial carcinoma in patient affected by multiple sclerosis: the first case in literature
Marco Marinaccio1*, Clark Christopher1, Porfido Valeria1, Carmen Zaza1, Giovanni Falcicchio1, Roberta Pellicciari2, Maria Luigia Mastronardi1, Alessia Giannoccaro1, Miriam Dellino1 and Ettore Cicinelli1
1Section of Gynecology and Obstetrics, Department of Biomedical Sciences and Human Oncology (DIMO), University of Bari “Aldo Moro”, 70124 Bari, Italy
2Department of Neurology “Luigi Amaducci”, University of Bari “Aldo Moro”, 70124 Bari, Italy
*Address for Correspondence: Marco Marinaccio, Section of Gynecology and Obstetrics, Department of Biomedical Sciences and Human Oncology (DIMO), University of Bari “Aldo Moro”, 70124 Bari, Italy, Email: [email protected]; [email protected]
Brain metastases in any gynecological cancer are a rare occurrence. Even more so, it is extremely rare for a gynecological malignancy to manifest itself with symptoms indicative of cerebral involvement. Literature regarding the association between MS and cancer is conflicting. We herein report a rare presentation of single metastasis of endometrial carcinoma in a 59-year-old woman affected by Primary Progressive Multiple Sclerosis (PPMS). A head CT scan was performed, which revealed the presence of an expansive lesion in the left parietal region. After careful assessment, a high-grade endometrial carcinoma was diagnosed and a decision was made to remove both the primary lesion and the brain metastasis in one sitting, through a conjoined surgery session involving neurosurgeons and gynecologists. The postoperative course was free from complications up until a few days after being transferred to a rehabilitation center, where she died following respiratory complications.
Multiple Sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS) and the leading non-traumatic cause of neurological disability in young adults. A recent study found that MS patients were associated with an increased risk of cancer compared to population controls, especially for respiratory organs (66%), CNS (52%), and urinary organs (51%) compared with a non-MS population [1]. A systematic review from 2015 found that the risk of gynecological cancer was the same or lower in the MS population [2]. The incidence of endometrial cancer was 0.65%; the risk of ovarian cancer ranged from 0.01% to 0.42% and finally, the risk of cervical cancer ranged from 0.01% to 0.56%. CNS metastases from any gynecological neoplasia are a rare occurrence and amount to about 1% of all tumors of uterine, cervical, or ovarian origin. Neoplasms originating from these sites, except Gestational Trophoblastic Neoplasms (GTN), are often considered “neurophobic” for this reason [3]. A recent retrospective study showed a possible increased risk of brain metastases for patients with type II endometrial cancers [4].
In a literature review, only seven cases of solitary brain metastasis preceding the diagnosis of uterine cancer have been documented [5]; in none of these cases, however, was the patient affected by MS: this might be the first case described in the literature of endometrial cancer manifesting itself with a single brain metastasis in a patient diagnosed with MS.
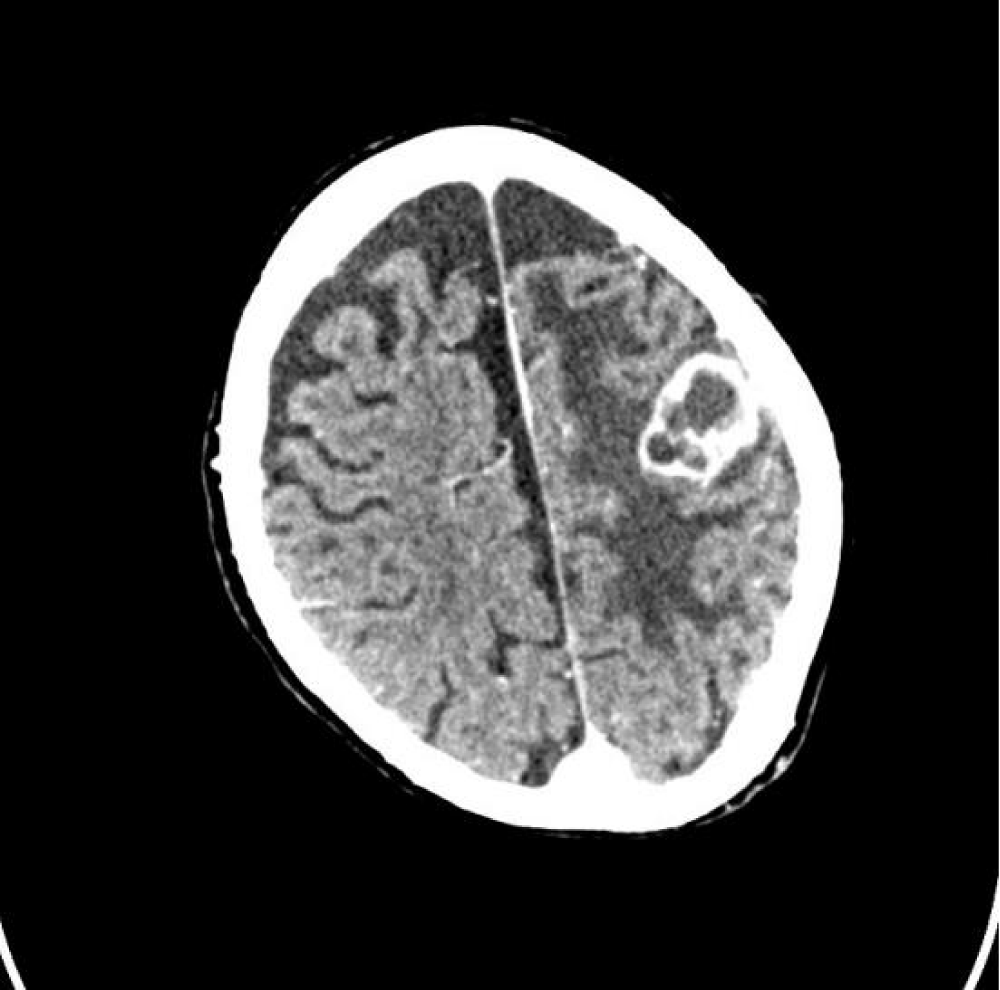
A 59-year-old woman, affected by primary progressive multiple sclerosis (PPMS), presented generalized weakness and clonus of the upper right limb that lasted about 2-3 minutes and resolved spontaneously in August 2022. During the following week, the patient showed worsening dysarthria, hypoesthesia of the lower right limb, and tonic-clonic seizures. Following this, she was taken to the Emergency Room of the Bari Policlinico. She also referred to hemorrhagic vaginal discharge in the last few years, which was never subjected to diagnostic investigation. A head computed-tomographic (CT) scan revealed an expansive lesion (24×27×28 mm) in the left parietal area with wide perilesional edema. The patient was subsequently admitted to the neurology department, she was unable to walk at the time of admission. She was oriented in time and space. She was affected by severe right hemiparesis and she suffered from urinary incontinence. The speech was clearly impaired. The patient underwent a total body CT scan, magnetic resonance imaging (MRI) of the head and pelvis and PET-CT scan. MRI scans confirmed the presence of said lesion in the left parietal area (Figure 1).
Figure 1a,b: Head MRI scan showing the presence of a single left parietal lesion with perilesional oedema.
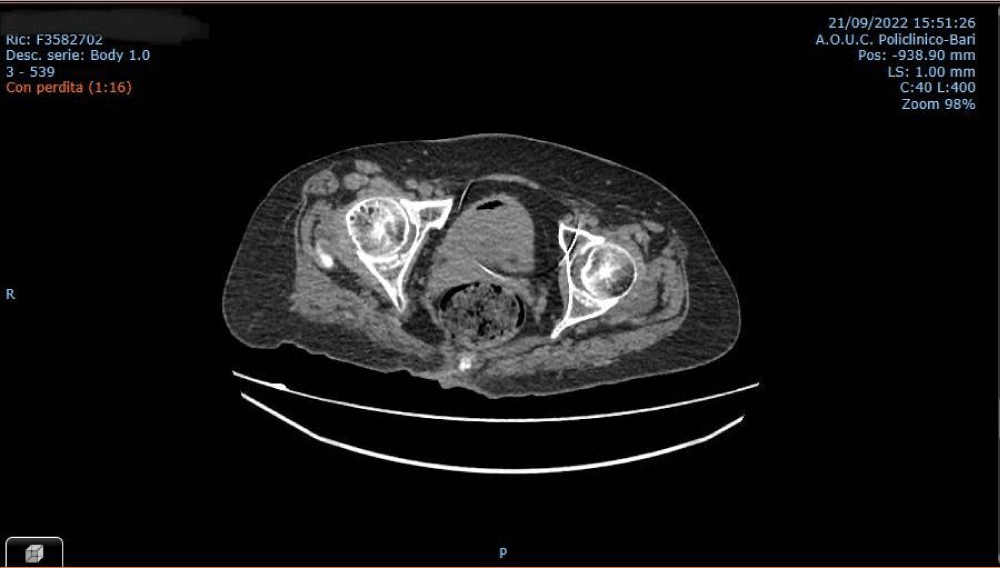
A voluminous pelvic lesion (maximum diameter 16 cm) possibly pertaining to the uterus which compressed and dislocated ileal loops, sigmoid colon, cecum, and the appendix was also found (Figure 2). Radiopharmaceutical accumulation in the pelvic and left parietal area was also detected. The patient subsequently underwent hysteroscopy, during which biopsies of the endometrium were taken. Histological examination revealed high-grade endometrial adenocarcinoma. Immunohistochemistry showed positivity of CKAE1/AE3, negative CD10 and AML, with wild-type p53. As the patient was stable due to her underlying pathology and having gone to the hospital due to a sudden worsening of neurological symptoms not related to multiple sclerosis, after a careful assessment of the patient’s condition, gynecologists and neurosurgeons decided to take her to the OR in order to remove the brain metastasis and pelvic mass in a single surgery session, because the expected benefits were clearly higher than the risk associated with surgery. In October 2022, the patient underwent a total hysterectomy, bilateral adnexectomy, and pelvic lymphadenectomy, followed by craniotomy with complete resection of the brain lesion. Following the surgery, histological examination of the uterus and adnexa revealed high-grade, poorly differentiated (G3) endometrioid carcinoma of the endometrium, infiltrating 50% of the myometrial layer, extended to the uterine isthmus. Immunohistochemistry showed positivity for CK8/18, focal positivity of CK7 and ER, negative Chromogranin, Synaptophysin, and PgR and wild-type expression of p53. Ki-67 proliferation index was about 85%. Diffuse LVSI was also evident, although histological examination of the left and right external iliac lymph nodes and of the superficial left obturator lymph nodes showed an absence of disease. Histological examination of the brain lesion confirmed this to be of endometrial origin. The postoperative course was free from complications and the patient was transferred to a rehabilitation center after 12 days. Sadly, the patient died shortly after due to a pulmonary embolism.
Figure 2: Pelvic CT-scan showing the uterine mass.
CNS metastases from endometrial neoplasia are rare and the extreme uniqueness of this case is represented by the fact that when brain metastasis was diagnosed in our patient, she did not show extracranial disease and the brain was the only site of systemic spread. Indeed, the definitive histological exam of the pelvic mass confirmed it to be a high-grade endometrial adenocarcinoma with a diffuse lymph-vascular invasion but without nodal involvement. We cannot define whether the onset of the patient’s symptoms was gradual or sudden, due to the underlying pathology, i.e., multiple sclerosis diagnosed in 1994. In fact, she presented spastic tetraparesis, especially pertaining to the left lower limb, dysphagia, and internuclear ophthalmoparesis for several years. She has been treated with azathioprine 50 mg twice per day, Gabapentin 400 mg 3 times per day, Clonazepam 2,5 mg/mL 8 gtt twice per day, and Bacoflen 3 mg per day. Azathioprine is carcinogenic to humans (Group 1) as reported in IARC Monograph. An increased risk of cancer has been reported in patients treated with azathioprine [6]. There were significantly increased risks of urinary tract cancer and lymphoid tissue cancer associated with azathioprine use. There were no other significant associations, but an elevated rate ratio with the lower boundary of the confidence interval just below 1 was observed for the subgroup of cancer of the female genital organs [7]. An early event that occurs in MS is the reduced functioning of the blood-brain barrier (BBB) which consists of specialized brain endothelial cells (BECs) that are supported in their barrier function by surrounding glial cells. Leakage and inflammation of the BECs in MS patients facilitate the massive influx of leukocytes into the brain parenchyma, which in turn induces irreversible demyelination, tissue damage, and axonal dysfunction [8]. The immune system undoubtedly plays an important role in MS; on the other hand, the role of the immune system in the onset of cancer has been extensively investigated. Immune system activation in patients suffering from autoimmune diseases might be crucial in protecting them from cancer by means of increased immunosurveillance; on the other hand, however, chronic inflammation resulting from immune system activation in autoimmune diseases could result in the activation of pro-tumorigenic pathways [9]. This dichotomy is reflected in the conflicting results found in the literature regarding the association between MS and cancer: some papers assert that patients with MS do not show an increased risk of cancer when compared to the control population [10], while other Authors conclude that patients with MS have higher cancer risk associated with previous use of immune suppressor therapy [11]. The BBB and the blood-cerebrospinal fluid barrier (BCB) represent a complex vasculature network that forms a continuous cellular barrier between the CNS and systemic circulation. Most of the important metabolic exchanges critical to CNS homeostasis occur through this tightly regulated network. Changes in its delicate balance have been associated with CNS pathologies such as MS [12]. It is believed that the inflammatory environment in demyelinating lesions leads to the generation of oxygen and nitrogen free radicals as well as proinflammatory cytokines, which contribute to the development and progression of the disease. Inflammation can lead to oxidative stress and vice versa. Thus, oxidative stress and inflammation are involved in a self-sustaining cycle [13]. The BBB has a dual role in brain metastasis formation: it forms a tight barrier protecting the central nervous system from entering cancer cells, but it is also actively involved in protecting metastatic cells during extravasation and proliferation in the brain [14]. These might be crucial clues in understanding the reason why a patient with a neurological disease such as MS developed a single brain metastasis that bypassed all the other more common sites of systemic spread.
CNS metastases from endometrial neoplasia are rare and the cases in which a solitary brain metastasis precedes the diagnosis of uterine cancers are even rarer. Among this already small group of cases, this is the first one in which the clinical presentation of endometrial cancer has been linked to a single metastasis localized within the CNS in a patient affected by MS. The impairment of the BBB in MS probably leads to the formation of brain metastasis, due to lower efficiency of the barrier in protecting against their formation. Another possible reason for this particular phenomenon may be that the inflammation and the release of pro-inflammatory cytokines led to a favorable environment in the homing process of the cancer cells, without the involvement of lymph nodes and other closer organs.
Author contribution
Conceptualization, MM, CC, VP and CZ, data curation, CC., VP., CZ, and RP; writing—original draft preparation, CC., VP., CZ, MD, and RP.; writing—review and editing, MM., MD., CC., VP., CZ, RP, GF and MLM.; visualization, MM.; supervision, MM., RP, MD., GF and MLM. All authors have read and agreed to the published version of the manuscript.
- Grytten N, Myhr KM, Celius EG, Benjaminsen E, Kampman M, Midgard R, Vatne A, Aarseth JH, Riise T, Torkildsen Ø. Risk of cancer among multiple sclerosis patients, siblings, and population controls: A prospective cohort study. Mult Scler. 2020 Oct;26(12):1569-1580. doi: 10.1177/1352458519877244. Epub 2019 Oct 1. PMID: 31573834.
- Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Sorensen PS, Reingold SC, Cutter G. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler. 2015 Mar;21(3):294-304. doi: 10.1177/1352458514564489. Epub 2014 Dec 22. PMID: 25533302; PMCID: PMC4429168.
- Hacker NF, Rao A. Surgical management of lung, liver and brain metastases from gynecological cancers: a literature review. Gynecol Oncol Res Pract. 2016 Jun 17;3:7. doi: 10.1186/s40661-016-0028-3. PMID: 27330821; PMCID: PMC4912748.
- Zhang Y, Grant MS, Stepp WH, Clark LH. Clinical characteristics of CNS metastases from primary gynecologic cancers. Gynecol Oncol Rep. 2019 Nov 11;30:100518. doi: 10.1016/j.gore.2019.100518. PMID: 31867433; PMCID: PMC6906733.
- Piura E, Piura B. Brain metastases from endometrial carcinoma. ISRN Oncol. 2012;2012:581749. doi: 10.5402/2012/581749. Epub 2012 Mar 18. PMID: 22523707; PMCID: PMC3316970.
- Confavreux C, Saddier P, Grimaud J, Moreau T, Adeleine P, Aimard G. Risk of cancer from azathioprine therapy in multiple sclerosis: a case-control study. Neurology. 1996 Jun;46(6):1607-12. doi: 10.1212/wnl.46.6.1607. PMID: 8649558.
- Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013 Jun 1;177(11):1296-305. doi: 10.1093/aje/kws375. Epub 2013 Mar 20. PMID: 23514635.
- Kamphuis WW, Derada Troletti C, Reijerkerk A, Romero IA, de Vries HE. The blood-brain barrier in multiple sclerosis: microRNAs as key regulators. CNS Neurol Disord Drug Targets. 2015;14(2):157-67. doi: 10.2174/1871527314666150116125246. PMID: 25613507.
- Melamed E, Lee MW. Multiple Sclerosis and Cancer: The Ying-Yang Effect of Disease Modifying Therapies. Front Immunol. 2020 Jan 10;10:2954. doi: 10.3389/fimmu.2019.02954. PMID: 31998289; PMCID: PMC6965059.
- Hongell K, Kurki S, Sumelahti ML, Soilu-Hänninen M. Risk of cancer among Finnish multiple sclerosis patients. Mult Scler Relat Disord. 2019 Oct;35:221-227. doi: 10.1016/j.msard.2019.08.005. Epub 2019 Aug 5. PMID: 31404761.
- Ragonese P, Aridon P, Vazzoler G, Mazzola MA, Lo Re V, Lo Re M, Realmuto S, Alessi S, D'Amelio M, Savettieri G, Salemi G. Association between multiple sclerosis, cancer risk, and immunosuppressant treatment: a cohort study. BMC Neurol. 2017 Aug 8;17(1):155. doi: 10.1186/s12883-017-0932-0. PMID: 28789625; PMCID: PMC5549380.
- Neumann H, Cavalié A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995 Jul 28;269(5223):549-52. doi: 10.1126/science.7624779. PMID: 7624779.
- Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, Hernández-Navarro VE, Sánchez-López AL, Alatorre-Jiménez MA. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. 2014 Nov;45(8):687-97. doi: 10.1016/j.arcmed.2014.11.013. Epub 2014 Nov 26. PMID: 25431839.
- Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. PMID: 23344048; PMCID: PMC3565326.