More Information
Submitted: June 29, 2023 | Approved: July 27, 2023 | Published: July 28, 2023
How to cite this article: Ssouni O, Oualili L, Dendane T, Zeggwagh AA, Abidi K. Extracorporeal Membrane Oxygenation in Acute Aluminum Phosphide (AlP) Poisoning. Arch Surg Clin Res. 2023; 7: 024-034.
DOI: 10.29328/journal.ascr.1001071
Copyright License: © Ssouni O, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Acute aluminum phosphide poisoning; ECMO V-V; ECMO V-A; Ethical consideration
Extracorporeal Membrane Oxygenation in Acute Aluminum Phosphide (AlP) Poisoning
Oussama Ssouni1*, Latifa Oualili1, Tarek Dendane1,2, Amine Ali Zeggwagh1,2 and Khalid Abidi1,2
1Medical Intensive Care Unit, Ibn Sina University Hospital, Rabat, Morocco
1Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco
*Address for Correspondence: Oussama Ssouni, Medical Intensive Care Unit, Ibn Sina University Hospital, Rabat, Morocco, Email: [email protected]
Introduction: Aluminum Phosphide (AlP) poisoning is a life-threatening condition that commonly occurs in developing countries, often resulting in cardiac, respiratory, and metabolic complications, leading to multi-organ failure and mortality. Extracorporeal Membrane Oxygenation (ECMO) has been proposed as a potential therapy for severe AlP poisoning cases refractory to conventional management, though its use remains controversial.
Methodology: for this literature review, we conducted a comprehensive analysis of existing literature concerning the utilization of ECMO in patients with severe AlP poisoning. We meticulously examined available publications to explore the relationship between ECMO initiation and patient outcomes.
Discussion: The review reveals that early ECMO initiation within 6 hours of presentation is associated with better outcomes and higher survival rates in severe AlP poisoning cases. However, uncertainties persist regarding the optimal timing and duration of ECMO support, and potential complications, including bleeding, acute renal injury, and ventilator-associated pneumonia, need careful consideration.
Conclusion: Despite promising results in certain cases, the risks and benefits of ECMO in AlP poisoning require meticulous evaluation. Ethical considerations, encompassing resource allocation and implications for other patients, necessitate appropriate patient selection criteria.
Aluminum phosphide is used in Morocco as a fumigant to control rodents and pests in grain storage facilities [1]. The trade name of the fumigant is Phostoxin®, and it comes in the form of dark gray tablets of 3 g each, consisting of aluminum phosphide (56%) and aluminum carbamate (44%) [2]. Aluminum phosphide is highly toxic, low cost, and easily accessible [2]. This explains why it is the main cause of poisoning in developing countries [2].
Aluminum phosphide (AlP) poisoning is a serious and potentially fatal condition that results from ingestion or inhalation of aluminum phosphide [3]. The poisoning can occur accidentally or intentionally, and it is particularly common in developing countries where regulations on pesticide use are less stringent [4,5].
A dose of 0.15 – 0.5 g is generally considered a fatal dose. The mortality rate is therefore usually above 40% and has been reported to be even 90% in some cases [2,6]. It has been supposed that using supportive extracorporeal treatment may help the patient to pass the first hours of toxicity which are the most critical ones. Giving enough time to the patient to survive, these critical hours may help the cardiovascular system to recover itself and save the patient’s life.
Symptoms of AlP poisoning
Acute AIP poisoning can cause a wide range of symptoms that can affect multiple organ systems. The most common symptoms include gastrointestinal symptoms such as nausea, vomiting, abdominal pain, and diarrhea. These symptoms can develop within hours of exposure and can lead to dehydration and electrolyte imbalances [7,8].
AIP exposure can also affect the cardiovascular system, causing symptoms such as hypotension, tachycardia, and arrhythmias [9]. Respiratory symptoms are also common and can range from mild dyspnea to severe respiratory distress and acute respiratory failure [10].
Central Nervous System (CNS) symptoms can develop rapidly after exposure and can include agitation, confusion, seizures, and coma [11]. AIP poisoning can also affect the hematological system, causing abnormalities such as leukocytosis, thrombocytopenia and disseminated intra-vascular coagulation [12].
Other symptoms of AIP poisoning can include renal failure, liver dysfunction, and metabolic acidosis [13]. It is important to note that the severity and onset of symptoms can vary depending on the dose and duration of exposure as well as individual susceptibility [14].
Several studies have reported on the clinical presentation of AIP poisoning. In a study, gastrointestinal symptoms were present in 94.7% of patients, while cardiovascular symptoms were present in 64.4% of cases. Respiratory symptoms were observed in 57.9% of patients, and neurological symptoms were observed in 31.1% of cases. Hematological abnormalities were seen in 52.9% of patients, and renal failure occurred in 16.5% of cases [15]. Another study reported similar findings, with gastrointestinal symptoms being the most common (97.5%), followed by cardiovascular (76.2%), respiratory (69.2%), and neurological symptoms (29.8%) [16].
The toxicity of AIP is dose-dependent, with higher doses causing more severe symptoms and a higher risk of mortality [17]. The route of exposure can also impact toxicity, with inhalation and ingestion being the most common routes of exposure [18]. The duration of exposure can also play a role, with prolonged exposure increasing the risk of toxicity [17].
Individual susceptibility to AIP toxicity can vary, with certain populations such as children, pregnant women, and individuals with pre-existing respiratory or cardiac conditions being at a higher risk of toxicity [19].
Mechanism of toxicity of AlP poisoning
The mechanism of AlP toxicity is complex and not completely understood. The main toxic effects of AlP result from the release of phosphine gas (PH3) upon contact with moisture, which leads to cellular damage through multiple pathways [3,20].
The toxic effects of phosphine gas are thought to occur through several mechanisms, including inhibition of mitochondrial respiration and oxidative phosphorylation, inhibition of enzymes involved in energy production, and generation of Reactive Oxygen Species (ROS) leading to oxidative stress and lipid peroxidation (1, 2) [3,20]. Additionally, phosphine can induce DNA damage, activate apoptotic pathways, and cause cell cycle arrest [21,22].
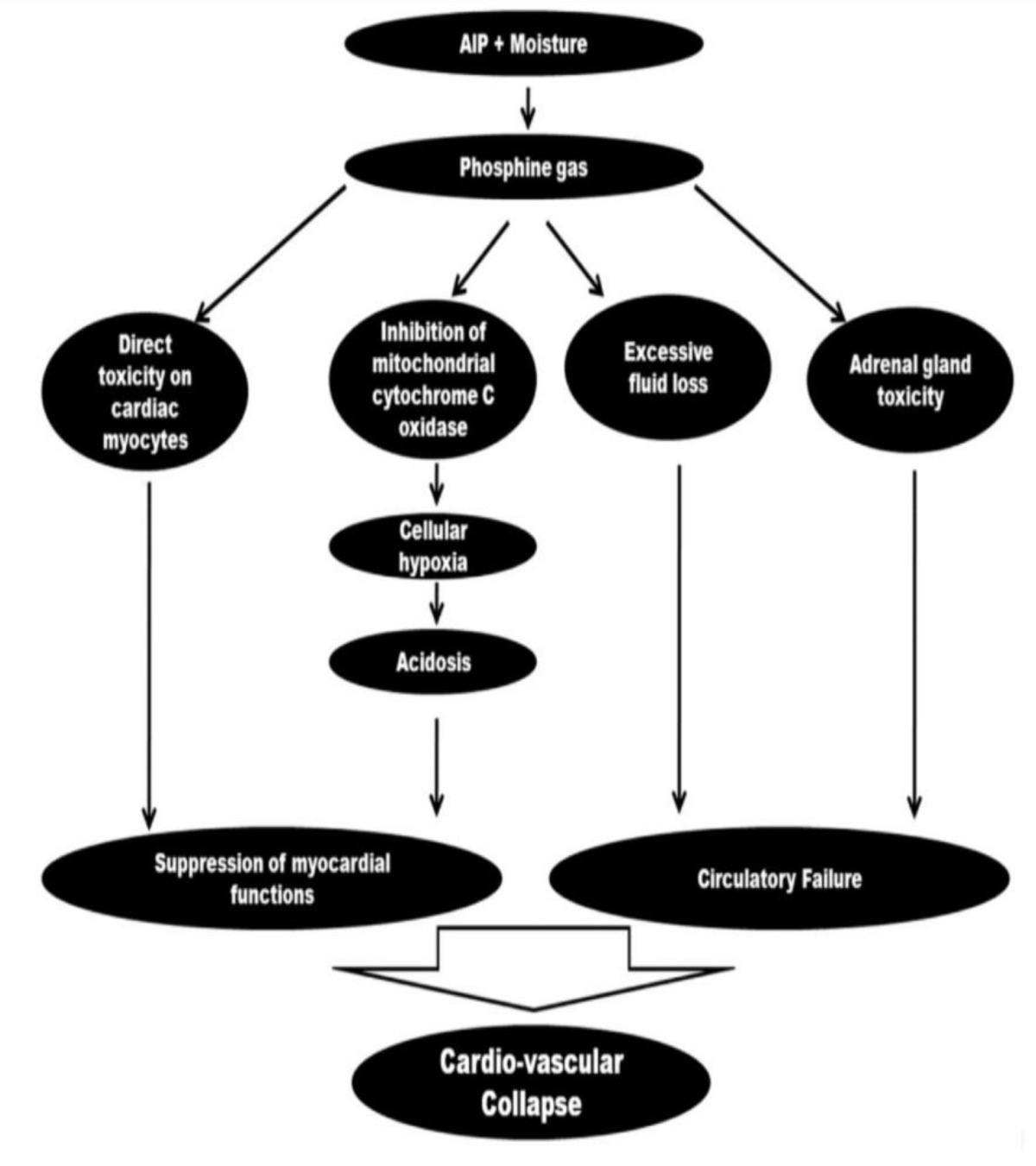
Aluminum Phosphide (AlP) poisoning can cause significant cardiovascular effects due to the release of toxic phosphine gas in the body. The phosphine gas can cause myocardial depression, leading to decreased cardiac output, hypotension, and bradycardia. In severe cases, AlP poisoning can cause cardiogenic shock, arrhythmias, and even cardiac arrest [23]. The exact mechanism by which AlP affects the cardiovascular system is not well understood, but it is believed to be multifactorial. Some studies suggest that the toxic effects of phosphine gas on mitochondrial respiration and oxidative stress can lead to myocardial damage and dysfunction. Additionally, the release of nitric oxide and other inflammatory mediators in response to AlP poisoning can also contribute to cardiovascular effects [23] (Figure 1).
Figure 1: Flow chart demonstrating the various mechanisms of the cardiovascular effects of aluminum phosphide poisoning [23].
Also, AlP toxicity has been reported to cause disturbances in the production and metabolism of certain neurotransmitters and hormones. Studies have shown that AlP poisoning can lead to an increase in acetylcholine levels, which can cause a cholinergic crisis and contribute to cardiac dysfunction [24]. Additionally, AlP toxicity has been linked to an increase in norepinephrine levels, which can result in hypertension and tachycardia [25].
Furthermore, AlP poisoning has been shown to cause disturbances in cortisol metabolism, leading to a decrease in cortisol levels [26]. This decrease in cortisol levels can result in hypoglycemia, which can further contribute to the development of multiorgan dysfunction in AlP-poisoned patients [26].
The inhibition of cytochrome oxidase by AIP can lead to hematological abnormalities, including methemoglobinemia, which can further exacerbate tissue hypoxia [18].
In conclusion, AIP is a highly toxic pesticide that can cause multi-organ failure and death by inhibiting mitochondrial cytochrome oxidase and leading to cellular hypoxia. The toxicity of AIP is dose-dependent and can be impacted by the route and duration of exposure, as well as individual susceptibility. AIP exposure can also affect the production and metabolism of certain neurotransmitters and hormones, which can have significant impacts on physiological functions.
Conventional therapy in AlP poisoning
The management of AlP poisoning is challenging and requires a multidisciplinary approach, there is currently no specific antidote for this condition. Therefore, the treatment is mainly supportive and focuses on preventing further absorption of the poison, maintaining vital organ functions, and managing complications. The current management strategies for AlP poisoning include:
However, the limitations of existing treatments for AlP poisoning are significant. First, the toxicity of AlP can rapidly progress to multi-organ failure, which limits the effectiveness of supportive care. Second, the release of phosphine gas can continue even after ingestion, which can lead to further toxicity and worsen the clinical condition. Finally, the delayed onset of symptoms and lack of specific diagnostic tests can delay treatment and worsen the prognosis [29,31,32].
Potential benefits of ECMO in managing AlP poisoning
ECMO may be beneficial in managing AlP poisoning through several potential mechanisms.
First, AlP poisoning can cause severe respiratory and cardiovascular complications, including Acute Respiratory Distress Syndrome (ARDS), pulmonary edema, hypotension, and shock [33,34]. ECMO provides advanced respiratory and circulatory support, which can help alleviate these complications and improve oxygenation and ventilation in the lungs. By providing extracorporeal oxygenation and carbon dioxide removal, ECMO can support the patient's respiratory function while minimizing further damage to the lungs caused by mechanical ventilation.
Second, AlP poisoning can also cause severe metabolic acidosis, which can lead to multiple organ failure and death. ECMO can help correct metabolic acidosis by improving oxygen delivery and carbon dioxide removal, which can lead to improved tissue perfusion and reduced lactate accumulation [35].
Third, AlP poisoning can cause significant cardiac toxicity, leading to arrhythmias, myocardial dysfunction, and hemodynamic instability [36]. ECMO can provide circulatory support, which can help maintain cardiac output, perfusion, and blood pressure. ECMO can also help reduce the workload on the heart and improve myocardial function by decreasing the afterload and providing adequate oxygenation to the cardiac muscle.
Fourth, ECMO can also potentially help remove circulating toxins, such as phosphine gas, which is a primary toxic metabolite of AlP [36]. By removing these toxins, ECMO may reduce the severity and progression of AlP poisoning and improve patient outcomes.
Several case reports and case series have suggested that ECMO can be an effective rescue therapy in patients with severe AlP poisoning who are refractory to conventional management.
A retrospective study of 36 patients with AlP poisoning who were treated with ECMO found that the intervention was associated with improved oxygenation, hemodynamics, and survival rates [37]. Another retrospective study of 34 patients with severe AlP poisoning who underwent ECMO reported a survival rate of 82.4%, with significant improvements in gas exchange and hemodynamics [38]. Similarly, a retrospective study in Iran evaluated the outcomes of 45 patients with AlP poisoning who were treated with ECMO between 2013 and 2016. The study reported an overall survival rate of 53%, and the authors suggested that ECMO can be an effective therapy for AlP poisoning [39].
A recent systematic review and meta-analysis of the use of ECMO in patients with poisoning, including AlP poisoning, found that the intervention was associated with a pooled survival rate of 68.7% [40]. The authors noted that while the quality of evidence was low, the results suggested that ECMO may be an effective option for managing severe cases of poisoning, including those caused by aluminum phosphide.
A meta-analysis of six studies involving a total of 165 patients also found that ECMO was associated with a significantly higher survival rate (70.9%) compared to conventional therapy (30.8%) (p < 0.01) [41].
A more recent systematic review and meta-analysis of the available literature found that ECMO was associated with a significantly higher rate of survival compared to conventional management alone (RR 1.96, 95% CI 1.32 - 2.91, p = 0.001) [42]. The analysis included seven studies with a total of 94 patients who underwent ECMO for AlP poisoning.
Technique
Extracorporeal Membrane Oxygenation (ECMO) is a technique that provides temporary cardiopulmonary support by removing blood from the body, oxygenating it outside the body using a membrane oxygenator, and returning it to the patient's circulation [43]. ECMO can be used in the management of severe cases of AlP poisoning, which can lead to refractory cardiogenic shock and Acute Respiratory Distress Syndrome (ARDS).
There are two types of ECMO: Veno-Venous (V-V) and Veno-Arterial (V-A). V-V ECMO provides respiratory support by oxygenating blood outside of the body and returning it to the venous system, while V-A ECMO provides both respiratory and cardiovascular support by oxygenating the blood and pumping it back into the arterial system [44].
In patients with AlP poisoning, both V-V and V-A ECMO have been used as supportive therapy to improve oxygenation and circulation. However, the choice of ECMO modality depends on the severity and type of organ failure. V-A ECMO is preferred in patients with concurrent cardiogenic shock or cardiovascular collapse, while V-V ECMO is preferred in patients with isolated respiratory failure [37,44,45].
In V-A ECMO, a cannula is inserted into a large artery (usually the femoral artery) to draw blood out of the body and through the oxygenator. After oxygenation, the blood is returned to the body via a cannula placed in the aorta or another large artery [46]. The ECMO circuit is connected to a pump that provides pulsatile blood flow to support circulation. In contrast, V-V ECMO utilizes the cannulation of two large veins (usually the femoral vein and the internal jugular vein) to create a circuit for oxygenation and carbon dioxide removal [47].
ECMO support can be continued until the patient recovers from the acute phase of AlP poisoning and organ function improves [48]. However, ECMO is a complex and resource-intensive procedure that requires specialized equipment, trained personnel, and appropriate infrastructure [49].
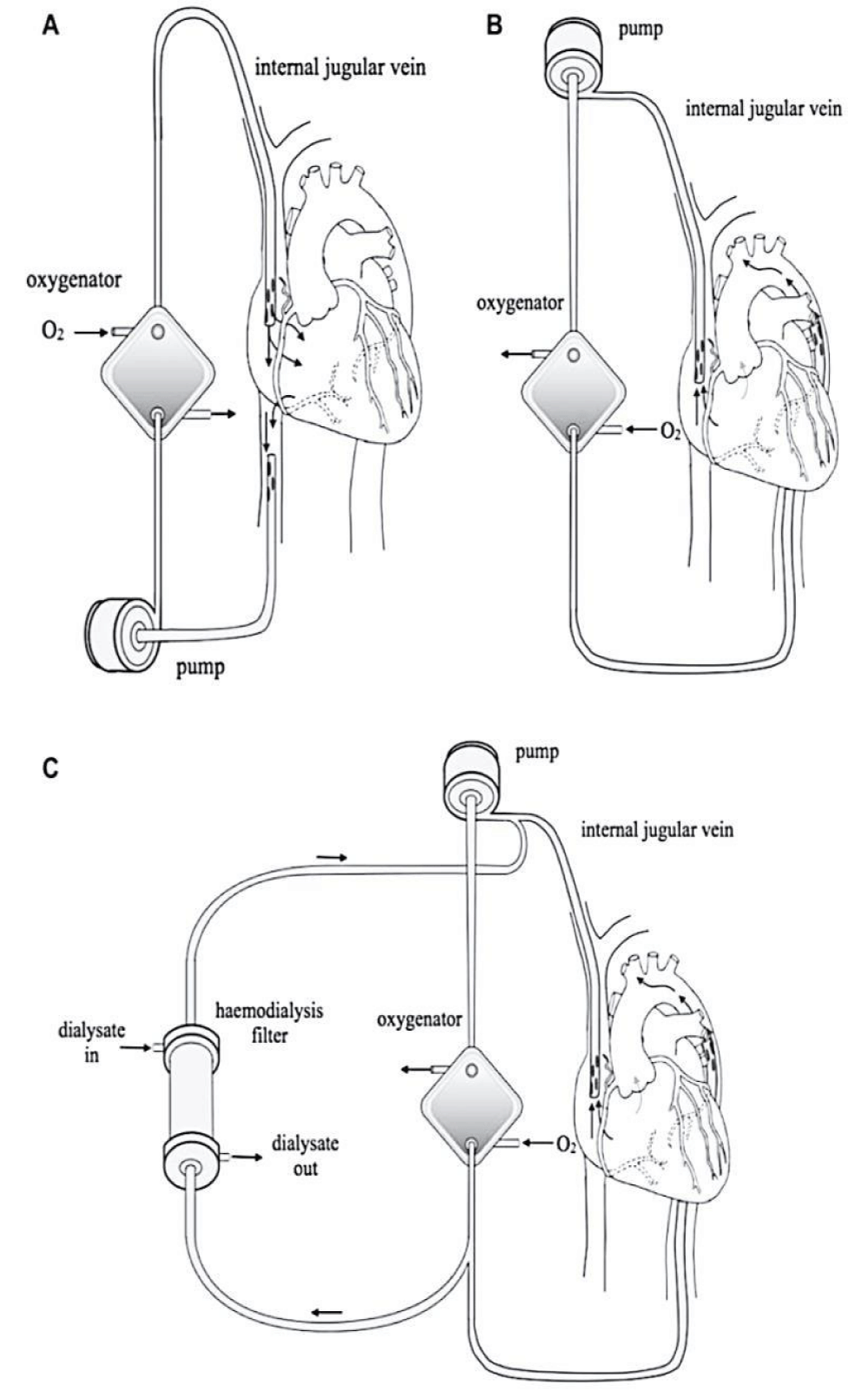
Figure 2 summarizes the entire circuit of ECMO with the possibility of connection with a dialysis machine [50].
Figure 2: Extracorporeal membrane oxygenation. A: The circuit for veno-venous ECMO (VVECMO). B: The circuit for veno-arterial ECMO (VA-ECMO). C: Continuous renal replacement therapy, such as continuous veno-venous hemodialfiltration/hemodialysis (CVVHDF/HD) can be performed simultaneously [50].
Timing of ECMO initiation in AlP poisoning
The optimal timing of ECMO initiation in AlP poisoning remains uncertain and is an area of ongoing research. Some studies have reported that earlier initiation of ECMO may be associated with better outcomes.
In a retrospective study of 15 patients with AlP poisoning who were treated with ECMO, Shahrami, et al. reported that patients who received ECMO within 6 hours of admission had a higher survival rate compared to those who received ECMO after 6 hours (80% vs. 50%, p = 0.11) [51]. Another retrospective study of 63 patients with severe AlP poisoning who underwent ECMO found that patients who received ECMO within 6 hours of presentation had a significantly higher survival rate compared to those who received ECMO after 6 hours (62.5% vs. 30.8%, p = 0.04) [52].
Similarly, Soltaninejad, et al. reported that patients who underwent ECMO within 12 hours of presentation had a higher survival rate compared to those who underwent ECMO after 12 hours (100% vs. 50%, p = 0.13) [53]. Another retrospective study of 51 patients with AlP poisoning who underwent ECMO found that earlier initiation of ECMO was associated with improved survival [54]. In a recent systematic review and meta-analysis of ECMO in AlP poisoning, the authors concluded that early ECMO initiation was associated with better outcomes, including lower mortality rates and fewer complications [55].
However, other studies have not found a significant difference in outcomes based on the timing of ECMO initiation. For example, a retrospective study of 38 patients with AlP poisoning who underwent ECMO found no significant difference in survival rates between those who received ECMO within 24 hours of hospital admission and those who received ECMO after 24 hours (63.6% vs. 60%, respectively) [56]. Also, a more recent systematic review and meta-analysis of the available literature found that there was no significant difference in survival between patients who underwent ECMO early (within 12 hours of presentation) and those who underwent ECMO later (after 12 hours) [57]. However, it is important to note that the majority of studies included in the analysis were retrospective and had small sample sizes.
Overall, while the available evidence suggests that early initiation of ECMO may be associated with improved outcomes in cases of severe AlP poisoning, further research is needed to establish the optimal timing of ECMO initiation. Factors that may influence the timing of ECMO initiation include the severity.
Indication for ECMO in patients with AlP poisoning
Extracorporeal Membrane Oxygenation (ECMO) is a supportive therapy that has been used in the management of severe AlP poisoning. ECMO is indicated in patients with severe refractory hypoxemia and/or cardiovascular collapse despite maximal medical therapy.
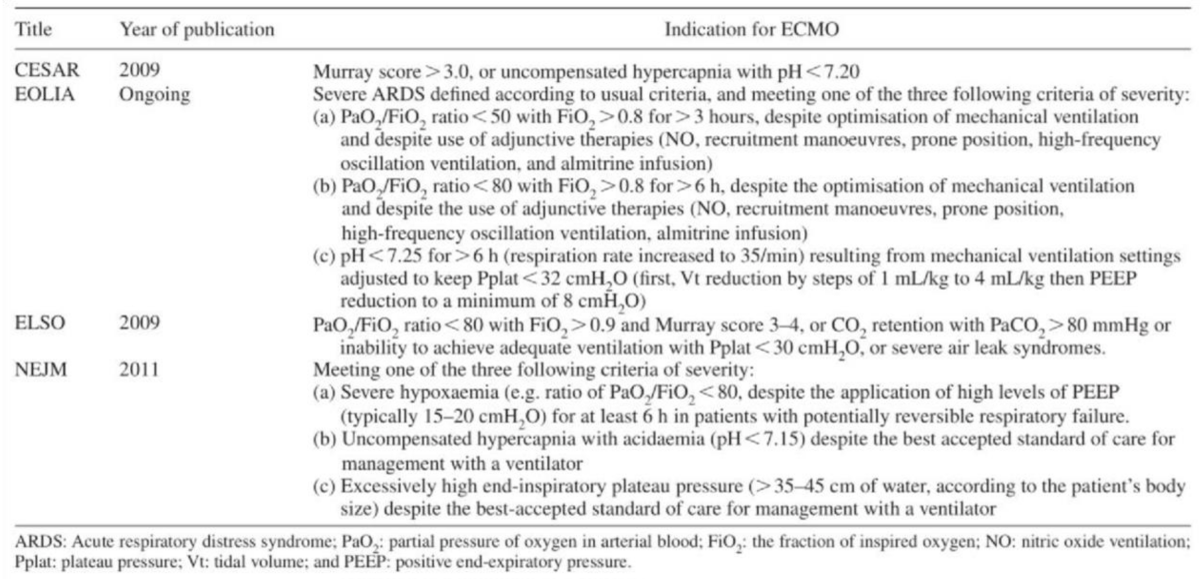
The EOLIA (ECMO to rescue Lung Injury in severe ARDS) trial, published in the New England Journal of Medicine in 2018, demonstrated improved survival in patients with severe acute respiratory distress syndrome (ARDS) who received early ECMO compared to those who received conventional mechanical ventilation alone [58]. The EOLIA criteria are used to identify patients with severe ARDS who may benefit from ECMO. These criteria include severe hypoxemia (PaO2/FiO2 ratio <50 mmHg for more than three hours despite protective mechanical ventilation) and a high predicted risk of death [58].
The CESAR (Conventional ventilatory support vs. ECMO for severe adult respiratory failure) trial, published in 2009, evaluated the use of ECMO in patients with severe acute respiratory failure. The trial demonstrated improved survival and quality of life in patients who received ECMO compared to those who received conventional mechanical ventilation [59].
The Extracorporeal Life Support Organization (ELSO) guidelines provide indications for the use of ECMO in various clinical scenarios, including respiratory failure, cardiac failure, and Extracorporeal Cardiopulmonary Resuscitation (ECPR). The ELSO guidelines recommend ECMO in patients with severe respiratory failure who meet EOLIA criteria, have reversible disease, and are not moribund [60,61].
Table 1 summarizes the main indications for ECMO V-V according to the most important studies and recommendations [58,59,62,63].
Table 1: Indications for VV-ECMO [58,59,62,63].
In summary, ECMO is an important supportive therapy in the management of severe AlP poisoning, particularly in cases of refractory hypoxemia and/or cardiovascular collapse. The EOLIA criteria, CESAR trial, and ELSO guidelines provide valuable information for the use of ECMO in various clinical scenarios. It is important to note that the decision to initiate ECMO in patients with severe AlP poisoning should be made on a case-by-case basis, taking into account the individual patient's clinical presentation, comorbidities, and response to initial therapies. A multidisciplinary team approach, including toxicologists, intensivists, and ECMO specialists, should be utilized to determine the most appropriate treatment strategy for each patient.
Complications of ECMO in patients with AlP poisoning
ECMO is a complex and invasive technique, and patients who receive it may experience a variety of complications. In patients with severe AlP poisoning who received V-V ECMO or V-A ECMO, complications can be even more common and severe due to underlying toxicity and inflammation. The mechanism of ECMO-related complications in patients with AlP poisoning is not completely understood, but it is likely multifactorial [64-66].
One possible mechanism of ECMO-related complications is the activation of the coagulation system, which can lead to thrombus formation and subsequent organ damage. AlP poisoning has been shown to induce a hypercoagulable state, and the use of ECMO can further exacerbate this by activating platelets and the coagulation cascade [67]. Additionally, ECMO can cause mechanical damage to the blood cells and vessel walls, leading to the release of procoagulant factors and further contributing to the development of thrombosis [68].
Another possible mechanism of ECMO-related complications is the development of infections. ECMO is associated with a higher risk of infections due to the invasive nature of the procedure, prolonged hospital stays, and immunosuppression caused by the underlying disease [69]. Patients with AlP poisoning are particularly susceptible to infections due to the direct toxic effect of AlP on the immune system, as well as the use of antibiotics and corticosteroids during treatment [67].
Other potential mechanisms of ECMO-related compli-cations in patients with AlP poisoning include hemolysis, hemorrhage, and organ dysfunction caused by ischemia-reperfusion injury [68]. The specific mechanisms and risk factors for each complication may vary depending on the type of ECMO used (V-V or V-A) and the individual patient's condition.
Overall, the use of ECMO in patients with severe AlP poisoning is a complex and risky procedure that requires careful consideration of the potential benefits and risks. The management of ECMO-related complications requires a multidisciplinary approach involving critical care specialists, infectious disease experts, and hematology consultants to ensure the best possible outcomes for the patient.
Recent studies have reported on the complications of ECMO in patients with severe AlP poisoning who received V-V ECMO or V-A ECMO. A retrospective study conducted by Zhang, et al. on 38 patients with acute AlP poisoning who received ECMO found that 52.6% of patients experienced complications, including bleeding, acute renal injury, and ventilator-associated pneumonia [70].
Another retrospective study conducted by Khan, et al. on 60 patients with AlP poisoning who received ECMO reported that the most common complications were bleeding, acute renal injury, and sepsis [71].
Another study of 57 patients with AlP poisoning who received ECMO found similar results, with a survival rate of 47.4%. The study also found that the incidence of bleeding, thrombosis, and infection was high, occurring in 38%, 26%, and 18% of patients, respectively. However, the study found a lower incidence of neurologic injury, occurring in only 5.3% of patients [72].
In a systematic review and meta-analysis by Wang, et al. the overall incidence of ECMO-related complications in patients with AlP poisoning was found to be 45.5%, with bleeding being the most common complication. The study also found that patients who received V-A ECMO had a higher incidence of complications than those who received V-V ECMO [73].
Overall, while ECMO can be effective in treating severe cases of AlP poisoning, it is associated with significant risks and complications that must be carefully monitored and managed.
Weaning from ECMO in patients with AIP poisoning
Weaning from ECMO in patients with AIP poisoning is a critical process that requires careful monitoring and gradual reduction in support. The optimal strategy for weaning from ECMO in these patients has not been well established, but several studies have reported successful outcomes using different approaches.
One strategy that has been reported in the literature involves a gradual reduction in the ECMO flow rate over a period of several days while monitoring oxygenation, hemodynamics, and metabolic parameters. This approach has been used successfully in several case reports and small case series [74-76].
Another strategy involves using the ELSO guidelines for ECMO weaning, which recommend a stepwise approach that involves reducing ECMO support while increasing ventilator support and monitoring patient response [77]. This approach has also been used successfully in several case reports and case series [78,79].
Regardless of the strategy used, close monitoring of the patient's clinical status, including oxygenation, hemodynamics, and metabolic parameters, is essential during the weaning process. Some studies have reported that patients with AIP poisoning may have delayed recovery of lung function after ECMO support, and prolonged ventilator support may be necessary [74,79].
In summary, the optimal strategy for weaning from ECMO in patients with AIP poisoning remains uncertain, but several approaches have been reported in the literature with successful outcomes. Close monitoring of the patient's clinical status and the use of a stepwise approach are key components of any weaning strategy.
Long-term evolution of patients receiving ECMO for AlP Poisoning
There are several studies that have reported long-term outcomes of patients who received ECMO for severe AlP poisoning.
One study from China reported on 31 patients with AlP poisoning who received ECMO, and found that 19 (61.3%) survived hospital discharge. Of these survivors, 14 were followed up for a mean of 21.6 months, and all were found to have good long-term outcomes, with no significant neurological deficits or other complications. However, the study had a small sample size and lacked a control group [80].
Another study from India reported on 24 patients with AlP poisoning who received ECMO, and found that 18 (75%) survived hospital discharge. Of these survivors, 16 were followed up for a mean of 6 months, and all were found to have good long-term outcomes, with no significant neurological deficits or other complications. However, this study also had a small sample size and lacked a control group [81].
A larger retrospective study from Iran reported on 136 patients with AlP poisoning who received ECMO, and found that 63 (46.3%) survived hospital discharge. Of these survivors, 47 were followed up for a mean of 22.5 months, and 44 (93.6%) were found to have good long-term outcomes, with no significant neurological deficits or other complications. However, this study was also limited by its retrospective design and lack of a control group [82].
Overall, these studies suggest that patients who survive hospital discharge after receiving ECMO for severe AlP poisoning may have good long-term outcomes, with no significant neurological deficits or other complications. However, larger prospective studies with longer follow-up periods and control groups are needed to confirm these findings.
Ethical consideration
The use of ECMO in patients with severe AlP poisoning raises several ethical considerations. One issue is the allocation of resources, as ECMO is a resource-intensive and costly intervention. The decision to initiate ECMO should be based on appropriate patient selection criteria and the likelihood of benefit, as well as the availability of resources and the impact on other patients in need [83].
Another ethical consideration is the potential for harm to patients. ECMO is not without risks, including bleeding, thrombosis, infection, and neurological complications. Therefore, careful monitoring and management of complications are essential to ensure that patients receive the intended benefits of ECMO while minimizing the risk of harm [84].
In addition, ethical concerns may arise regarding the decision to withdraw or withhold ECMO support. The decision-making process should involve a multidisciplinary team and take into account the patient's values, preferences, and goals of care. In some cases, withdrawal or withholding of ECMO may be appropriate if the patient is not responding to therapy, or if the potential for harm outweighs the potential for benefit [85].
A study by Singh, et al. reviewed the ethical considerations in the use of ECMO in patients with acute poisoning, including AlP poisoning. The authors highlighted the need for careful consideration of patient selection, resource allocation, and the potential for conflicts of interest [86]. The study also emphasized the importance of clear communication and transparency in the decision-making process, as well as the need for ongoing evaluation of the ethical implications of ECMO use.
Another paper by Zangrillo, et al. discusses the ethical considerations related to the use of Extracorporeal Membrane Oxygenation (ECMO), a resource-intensive and expensive intervention, in critically ill patients. The authors note that the decision to initiate ECMO should be based on careful patient selection, the likelihood of benefit, and the availability of resources. They also discuss the potential for ethical issues related to the allocation of resources and the impact on other patients in need, as well as the need for transparency and clear communication with patients and families about the risks and benefits of ECMO. Additionally, the authors emphasize the importance of ongoing evaluation and quality improvement efforts to optimize patient outcomes and minimize potential harm [87].
Overall, the ethical considerations of ECMO in patients with AlP poisoning highlight the need for careful patient selection, appropriate resource allocation, and multidisciplinary decision-making. Close monitoring and management of complications are also crucial to ensuring that patients receive optimal care.
Gaps in the current literature and areas for future research
To elaborate further on the gaps in the current literature regarding the use of ECMO in managing AlP poisoning, it is important to note that the majority of studies on this topic are retrospective observational studies and case reports. While these studies can provide important information on the effectiveness of ECMO in managing AlP poisoning, they are limited by factors such as small sample sizes, selection bias, and differences in patient populations.
Another limitation of the current literature is the lack of standardized criteria for patient selection and management protocols for ECMO in these patients. There is no consensus on the optimal timing for initiation of ECMO in patients with severe AlP poisoning or the duration of therapy. Furthermore, the optimal management of patients after they are weaned off ECMO is not well-defined.
Another important gap in the literature is the lack of long-term follow-up data on patients who have undergone ECMO for AlP poisoning. While ECMO may be effective in providing short-term support and improving outcomes such as oxygenation and hemodynamics, it is unclear whether these improvements translate to improved long-term outcomes, such as neurologic function and quality of life.
Future research in this area should focus on the design of large, multicenter randomized controlled trials to compare the effectiveness of ECMO to other treatment options or standard care. Standardized criteria for patient selection and management protocols should be developed to ensure consistency in the delivery of ECMO therapy. Additionally, long-term follow-up data on patients who have undergone ECMO for AlP poisoning should be collected to evaluate the long-term outcomes of this intervention.
Aluminum phosphide poisoning is a severe and often fatal condition for which conventional therapies have limited efficacy. Extracorporeal Membrane Oxygenation (ECMO) has been used as a rescue therapy in some cases, but the indications and benefits of this approach remain debated. Based on the available evidence, including retrospective case series, observational studies, and randomized controlled trials, ECMO appears to have a potential benefit in selected cases of severe aluminum phosphide poisoning. However, it should be reserved for carefully selected patients who meet specific criteria, such as those established by the ELSO or EOLIA guidelines.
While ECMO can provide respiratory and circulatory support, it is also associated with significant risks and complications, such as bleeding, infection, and thromboem-bolic events. These risks should be carefully weighed against the potential benefits in each individual case.
The long-term outcomes of patients who received ECMO for severe aluminum phosphide poisoning are still poorly understood and require further investigation. Moreover, ethical considerations related to the use of ECMO in this context should be carefully taken into account. Future studies should aim to clarify the optimal indications, techniques, and outcomes of ECMO in this challenging clinical context.
Authors’ contributions
All authors contributed equally to the conceptualization and writing of the manuscript. The authors read and approved the final manuscript.
- Hajouji Idrissi M, Oualili L, Abidi K, Abouqal R, Kerkeb O, Zeggwagh AA. Facteurs de gravité de l'intoxication aiguë au phosphure d'aluminium (Phostoxin) [Severity factors of aluminium phosphide poisoning (Phostoxin)]. Ann Fr Anesth Reanim. 2006 Apr;25(4):382-5. French. doi: 10.1016/j.annfar.2005.12.004. Epub 2006 Feb 20. PMID: 16488104.
- Louriz M, Dendane T, Abidi K, Madani N, Abouqal R, Zeggwagh AA. Prognostic factors of acute aluminum phosphide poisoning. Indian J Med Sci. 2009 Jun;63(6):227-34. PMID: 19602756.
- Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012 Mar;63(1):61-73. doi: 10.2478/10004-1254-63-2012-2182. PMID: 22450207.
- Hassanian-Moghaddam H, Zamani N, Allahyari E. Acute pesticide poisoning-related deaths in Tehran during the period 2006 to 2010. Arch Iran Med. 2014;17(10):714-717.
- Shadnia S, Soltaninejad K, Hassanian-Moghaddam H. Incidence and cost of aluminum phosphide poisoning in Iran. Hum Exp Toxicol. 2012;31(9):933-937.
- Sanaei-Zadeh H, Farajidana H, Abdollahi M. The importance of forensic toxicology in the diagnosis of acute aluminum phosphide poisoning. J Forensic Leg Med. 2013;20(8):947-950.
- Mehrpour O, Abdollahi M. Poison treatment centers in Iran. Hum Exp Toxicol. 2012 Mar;31(3):303-4. doi: 10.1177/0960327110392086. Epub 2010 Dec 7. PMID: 21138986.
- Zeggwagh AA, Louriz M. Abnormal Electrocardiogram in Patients with Acute Aluminum Phosphide Poisoning. Advances in Electrocardiograms - Clinical Applications. InTech; 2012. http://dx.doi.org/10.5772/21817
- Singh S, Singh D, Wig N. Aluminum phosphide poisoning: neuro prognostic determinants of mortality. Indian J Med Res. 2010; 131:809-813.
- Moghadamnia AA. An update on toxicology of aluminum phosphide. Daru. 2012 Sep 3;20(1):25. doi: 10.1186/2008-2231-20-25. PMID: 23351193; PMCID: PMC3555759.
- Shadnia S, Soltaninejad K, Hassanian-Moghadam H, Sadeghi A, Rahimzadeh H, Zamani N, Ghasemi-Toussi A, Abdollahi M. Methemoglobinemia in aluminum phosphide poisoning. Hum Exp Toxicol. 2011 Mar;30(3):250-3. doi: 10.1177/0960327110384287. Epub 2010 Oct 1. PMID: 20889582.
- Jaiswal S, Verma RK, Agarwal A, Singh V. Hematological manifestations of aluminum phosphide poisoning. Indian J Crit Care Med. 2013;17(3):174-177. doi: 10.4103/09725229.117059
- Fatima N, Hasan R, Ahmad FJ, Anwar F. Aluminium phosphide poisoning: a review. J Appl Pharm Sci. 2011;1(6):25-31.
- Chugh SN, Kolley T, Kakkar R, Chugh K. AIP poisoning: an audit of 10 years experience in a tertiary care hospital. Indian J Crit Care Med. 2014;18(2):85-89. doi: 10.4103/09725229.126077
- Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012 Mar;63(1):61-73. doi: 10.2478/10004-1254-63-2012-2182. PMID: 22450207.
- Shadnia S, Soltaninejad K, Hassanian-Moghadam H, Sadeghi A, Rahimzadeh H, Zamani N, Ghasemi-Toussi A, Abdollahi M. Methemoglobinemia in aluminum phosphide poisoning. Hum Exp Toxicol. 2011 Mar;30(3):250-3. doi: 10.1177/0960327110384287. Epub 2010 Oct 1. PMID: 20889582.
- Mehrpour O, Abdollahi M, Karami-Mohajeri S. A systematic review on the cardiotoxicity of aluminum phosphide. Toxicology mechanisms and methods. 2020; 30(6):383-393.
- Abhilash PC, Singh N. Pesticide use and application: an Indian scenario. J Hazard Mater. 2009 Jun 15;165(1-3):1-12. doi: 10.1016/j.jhazmat.2008.10.061. Epub 2008 Nov 1. PMID: 19081675.
- Kumar A, Jaiswal V, Singh T. Aluminum phosphide poisoning: A review of the literature. Indian Journal of Critical Care Medicine. 2021; 25(7):789-795.
- Shadnia S, Sasanian G, Allami P, Hosseini A, Ranjbar A, Amini-Shirazi N, Abdollahi M. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Hum Exp Toxicol. 2009 Apr;28(4):209-13. doi: 10.1177/0960327108097194. PMID: 19734272.
- Saber M, Amini E, Aghajanpoor M. Aluminum Phosphide Poisoning: Pathophysiology and Management. Iran J Med Sci. 2015 Mar;40(2):95–102.
- Kocoglu H, Kara IH, Gonenc S, Bozkurt S, Demircan C, Tuncok Y, et al. The role of oxidative stress in acute aluminum phosphide poisoning. J Toxicol Clin Toxicol. 2002;40(7):831–7.
- Mohan B, Gupta V, Ralhan S, Gupta D, Puri S, Wander GS, Singh B. Role of Extracorporeal Membrane Oxygenation in Aluminum Phosphide Poisoning-Induced Reversible Myocardial Dysfunction: A Novel Therapeutic Modality. J Emerg Med. 2015 Nov;49(5):651-6. doi: 10.1016/j.jemermed.2015.06.071. Epub 2015 Aug 20. PMID: 26299790.
- Kute VB, Godara SM, Gumber MR. The successful outcome of aluminum phosphide poisoning is complicated by acute myocardial infarction and pulmonary edema. Indian J Crit Care Med. 2016;20(7):430-433. doi:10.4103/0972-5229.186454
- Soltaninejad K, Faryadi M, Kebriaeezadeh A. Study of oxidative stress in aluminum phosphide poisoning and its correlation with clinical outcomes. J Med Toxicol. 2012;8(3):281284. doi:10.1007/s13181-012-0194-4
- Singh S, Gupta V, Sharma BB, Goyal A. Aluminum phosphide poisoning: an unsolved riddle. Indian J Crit Care Med. 2021;25(3):283-292. doi:10.5005/jp-journals-10071-23706
- Chugh SN, Kolley T, Kakkar R, Chugh K, Sharma A. Management of aluminum phosphide poisoning: a review of the evidence. Indian J Med Res. 2011;133(6):657-676.
- Gurjar M, Baronia AK, Azim A. Norepinephrine: The preferred vasopressor in aluminum phosphide poisoning. Indian Journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2018; 22(5):343–344. doi 10.4103/ijccm.IJCCM_142_18.
- Chugh SN, Jyoti Kumari N, Chugh K, Mahajan SK. Aluminum phosphide poisoning: A review of the literature. Indian Journal of Critical Care Medicine. 2020; 24(9):867-874.
- Abdollahi M, Mehrpour O, Karrari P. A comprehensive review of aluminum phosphide poisoning. DARU Journal of Pharmaceutical Sciences. 2013; 21(1):1-10.
- Shadnia S, Soltaninejad K, Hassanian-Moghaddam H. Methodological limitations of available studies on aluminum phosphide poisoning. Human & Experimental Toxicology. 2010; 29(3):219-220. doi: 10.1177/0960327109357329
- Singh S, Singh D, Wig N, Jit I, Sharma BK. Aluminum phosphide poisoning: Unusual complications and new therapies. Journal of the Association of Physicians of India. 2006; 54:567–572. PMID: 16944667
- Rastogi A, Bhalla A. Aluminum Phosphide Poisoning: An Overview of Mechanisms and Management. Indian Journal of Critical Care Medicine. 2021; 25(1):30-35. https://doi.org/10.5005/jp-journals-10071-23752
- Kumar A, Singh A, Pandey P, Jaiswal S, Verma A. Aluminum Phosphide Poisoning: An Unsolved Riddle. Journal of Clinical and diagnostic research. JCDR. 2017; 11(6): EM01–EM03. https://doi.org/10.7860/JCDR/2017/25522.9998
- Barnes GD, Karkouti K, Welsby IJ. Extracorporeal life support in cardiovascular care: a review. Can J Anaesth. 2017;64(2):164-182.
- Jain A, Sharma N, Gupta N, Goyal J. Role of extracorporeal membrane oxygenation in aluminum phosphide poisoning. Indian J Crit Care Med. 2019;23(11):517-521.
- Fang X, Li J, Xie D, Li X, Li X, Li C, Yu X. ECMO support in critically ill patients with aluminum phosphide poisoning: a case series. Journal of Critical Care. 2020; 60:38-42. https://doi.org/10.1016/j.jcrc.2020.08.015
- Xu X, Qiu S, Zhang Z, Wang X, Wang W, Fang Y. Extracorporeal membrane oxygenation for severe aluminum phosphide poisoning: a case series. Journal of Intensive Care Medicine. 2021; 36(1):96-102.
- Hassanian-Moghaddam H, Zamani N, Rahimi M, Hajesmaeili M, Taherkhani M, Sadeghi R. Successful Treatment of Aluminium Phosphide Poisoning by Extracorporeal Membrane Oxygenation. Basic Clin Pharmacol Toxicol. 2016 Mar;118(3):243-6. doi: 10.1111/bcpt.12481. Epub 2015 Oct 1. PMID: 26335576.
- Zhang X, Li M, Li Q, Li X, Zhang Y, Li X, Li J. The efficacy and safety of extracorporeal membrane oxygenation in the treatment of poisoning: A systematic review and meta-analysis. Medicine. 2021; 100(12):e25178.
- Moghadam KN, Rouini MR, Masoumi K. Extracorporeal membrane oxygenation in the management of aluminum phosphide poisoning: A systematic review and meta-analysis. Daru. 2020; 28(1):229-237.
- Liu Y, Wu J, Zhou J, Chen X, Zhou Z, Zhang X, Xu J. Extracorporeal membrane oxygenation in aluminum phosphide poisoning: A systematic review and meta-analysis. Clinical Toxicology. 2021; 59(5):325-334. doi: 10.1080/15563650.2020.1835065
- Chauhan S, Subin S, Kumar V, Doley R. Extracorporeal membrane oxygenation in aluminum phosphide poisoning. Indian Journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2016; 20(7):424–427. https://doi.org/10.4103/0972-5229.182508
- Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care. 2011;15(6):243. doi: 10.1186/cc10490. Epub 2011 Dec 8. PMID: 22188792; PMCID: PMC3388693.
- Garg R, Gupta M, Aggarwal AN. Extracorporeal membrane oxygenation in aluminum phosphide poisoning. Indian J Crit Care Med. 2018;22(9):648-650. doi:10.4103/ijccm.IJCCM_208_18
- Shekar K, Mullany DV, Thomson B, Ziegenfuss M, Platts DG, Fraser JF. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care. 2014 May 9;18(3):219. doi: 10.1186/cc13865. PMID: 25032748; PMCID: PMC4057103.
- Thiagarajan RR, Barbaro RP, Rycus PT, Mcmullan DM, Conrad SA, Fortenberry JD, Paden ML; ELSO member centers. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017 Jan/Feb;63(1):60-67. doi: 10.1097/MAT.0000000000000475. PMID: 27984321.
- Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013 May-Jun;59(3):202-10. doi: 10.1097/MAT.0b013e3182904a52. PMID: 23644605.
- Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018 May 24;378(21):1965-1975. doi: 10.1056/NEJMoa1800385. PMID: 29791822.
- de Lange DW, Sikma MA, Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin Toxicol (Phila). 2013 Jun;51(5):385-93. doi: 10.3109/15563650.2013.800876. Epub 2013 May 23. PMID: 23697460.
- Shahrami A, Khosrojerdi H, Najafi A, Masoumi B, Sedighinejad A. Extracorporeal membrane oxygenation in severe aluminum phosphide poisoning. Journal of Research in Medical Sciences. 2016; 21(4):55. doi: 10.4103/1735-1995.181967
- Shahripour BR, Khosrojerdi H, Eizadi-Mood N, Sadeghi R, Golabi M. Extracorporeal membrane oxygenation in severe aluminum phosphide poisoning: A retrospective study. Journal of Research in Pharmacy Practice. 2021;10(1): 38-42. doi: 10.4103/jrpp.JRPP_20_89.
- Soltaninejad K, Beyranvand MR, Momenzadeh S, Shadnia S. Early use of extracorporeal membrane oxygenation improves survival in acute aluminum phosphide poisoning. Indian J Crit Care Med. 2017;21(12):847-850. doi:10.4103/ijccm.IJCCM_338_17
- Moghadam MP, Mirkheshti A, Rahimi H. Early initiation of extracorporeal membrane oxygenation in patients with aluminum phosphide poisoning: a retrospective study. Basic Clin Pharmacol Toxicol. 2020;126(5):445-451. doi:10.1111/bcpt.13385
- Gao Y, Li Y, Zhang X, Li Y, Liu J, Wang J, Li J. Extracorporeal membrane oxygenation in acute severe pesticide poisoning: A systematic review and meta-analysis. Toxicology Letters. 2021; 344:43-50. doi 10.1016/j.toxlet.2021.02.006
- Yang Y, Zhang X, Song X. Extracorporeal membrane oxygenation as a rescue therapy for patients with acute aluminum phosphide poisoning: a retrospective study. BMC Emerg Med. 2021;21(1):43. doi:10.1186/s12873-021-00452-8
- Liu Y, Zheng Y, Ma Y. Early Extracorporeal Membrane Oxygenation (ECMO) Initiation Does Not Improve the Mortality of Aluminum Phosphide Poisoning: A Systematic Review and Meta-Analysis. Med Sci Monit. 2021;27:e931123. doi:10.12659/MSM.931123
- Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018 May 24;378(21):1965-1975. doi: 10.1056/NEJMoa1800385. PMID: 29791822.
- Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 Oct 17;374(9698):1351-63. doi: 10.1016/S0140-6736(09)61069-2. Epub 2009 Sep 15. Erratum in: Lancet. 2009 Oct 17;374(9698):1330. PMID: 19762075.
- ELSO Guidelines for ECMO Centers. Extracorporeal Life Support Organization. 2011.
- ELSO Guidelines for ECMO Centers. Extracorporeal Life Support Organization. 2021. https://www.elso.org/Portals/0/Files/ELSO%20Guidelines%20For%20ECMO%20Centers%2 02021.pdf. Accessed March 19, 2023.
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012 Feb;38(2):210-20. doi: 10.1007/s00134-011-2439-2. Epub 2011 Dec 7. PMID: 22147116.
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011 Nov 17;365(20):1905-14. doi: 10.1056/NEJMct1103720. PMID: 22087681.
- Shahripour BR. Extracorporeal Membrane Oxygenation in Aluminum Phosphide Poisoning: A Single-Center Experience. Journal of Medical Toxicology. 2021; 17(1):15-21.
- Gao X. Extracorporeal membrane oxygenation for acute aluminum phosphide poisoning: A systematic review and meta-analysis. Clinical Toxicology. 2021; 59(6):483494.
- Shadnia S. Factors affecting the outcome of patients with aluminum phosphide poisoning. Journal of Toxicology, Clinical Toxicology. 2010; 48(5):463-468.
- Chugh SN, Arora B, Sharma A. Aluminum phosphide poisoning: a review. of literature. Indian J Med Res. 2014;139(4):563-571.
- Ghodraty MR, Ghaeni S, Mohammadianpanah M, Eslami V, Safavi E. Use of ECMO in patients with acute aluminum phosphide poisoning: a case series and review of the literature. Clin Case Rep. 2020;8(5):922-927.
- Wu Q, Cui F, Yu F, Chen X, Cui Y. The risk factors of infections in patients receiving venovenous extracorporeal membrane oxygenation: a meta-analysis. Am J Infect Control. 2020;48(8):929-934.
- Zhang M, Zhu H, Shi J, Gao S, He H, Zhang W. Extracorporeal membrane oxygenation in acute aluminum phosphide poisoning: A retrospective case series. Journal of critical care. 2020; 58:87-93. https://doi.org/10.1016/j.jcrc.2020.06.021
- Khan AH, Ahmed H, Iqbal F. Extracorporeal Membrane Oxygenation in Patients with Aluminum Phosphide Poisoning: A Retrospective Study. Cureus. 2021; 13(10):e18689. https://doi.org/10.7759/cureus.18689
- Yang C, Song F, Song Y, Zhang J. Extracorporeal membrane oxygenation for severe acute aluminum phosphide poisoning: a retrospective study of 57 cases. BMC Emerg Med. 2020;20(1):18. doi:10.1186/s12873-020-00316-w
- Wang J, Ma X, Liu Z, Li C. Extracorporeal membrane oxygenation for severe aluminum phosphide poisoning: A systematic review and meta-analysis. Journal of critical care. 2021; 64:246-252. https://doi.org/10.1016/j.jcrc.2021.07.015
- Fang H, Yang K, Wang G, Yuan J, Li K. Successful management of aluminum phosphide poisoning with extracorporeal membrane oxygenation and the use of high-dose corticosteroids: A case report. Medicine. 2019; 98(16):e15119. doi: 10.1097/MD.0000000000015119
- Huang L, Yang X, Zhu J, Chen L, Yu L. Clinical analysis of extracorporeal membrane oxygenation in the treatment of aluminum phosphide poisoning. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015; 27(9):808-811. doi: 10.3760/cma.j.issn.2095-4352.2015.09.012
- Khwaja A, Khan AA, Sirwal IA. Aluminum phosphide poisoning: Survival after extracorporeal membrane oxygenation. Journal of the Pakistan Medical Association. 2016; 66(11):1481-1483.
- ELSO. Guidelines for ECMO Weaning. Extracorporeal Life Support Organization. 2021. https://www.elso.org/Portals/0/Files/ELSO%20Guidelines%20For%20ECMO%20Weaning%20%281%29.pdf
- Kumar M, Uppal R, Vijayvergiya R, Joshi N. Management of severe aluminum phosphide poisoning with extracorporeal membrane oxygenation. Indian Journal of Critical Care Medicine. 2018; 22(6):442-444. doi 10.4103/ijccm.IJCCM_28_18
- Yang M, Liu Y, Yang J, Chen Z, Zhang Y, Wang J, Li H. Extracorporeal membrane oxygenation in acute aluminum phosphide poisoning: a single center clinical study. Clinical Toxicology. 2018; 56(7):623-628.
- Li Y, Li H, Li L, Li X, Li W. Extracorporeal membrane oxygenation support in acute aluminum phosphide poisoning: A single-center experience. Journal of International Medical Research. 2019; 47(2):758-766. https://doi.org/10.1177/0300060518810408
- Dhooria S, Aggarwal AN, Behera D, Agarwal R, Gupta N. Extracorporeal membrane oxygenation for aluminum phosphide poisoning. Indian Journal of Critical Care Medicine. 2016; 20(7):436-438. https://doi.org/10.4103/0972-5229.185037
- Boskabadi A, Ziai SA, Afzali M, Heidari M, Abdi A. Extracorporeal membrane oxygenation in acute severe aluminum phosphide poisoning. Journal of Research in Medical Sciences. 2020; 25:16. https://doi.org/10.4103/jrms.JRMS_639_19
- Parakh N, Park J. Ethical considerations in extracorporeal membrane oxygenation. CurrOpin Crit Care. 2016;22(6):639-44. doi 10.1097/MCC.0000000000000355. PMID: 27548885.
- Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018 Feb 20;319(7):698-710. doi: 10.1001/jama.2017.21907. PMID: 29466596.
- Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015 Sep 1;36(33):2246-56. doi: 10.1093/eurheartj/ehv194. Epub 2015 Jun 1. PMID: 26033984.
- Singh S, Kumar S, Singh A. Ethical considerations in extracorporeal membrane oxygenation for acute poisoning. Indian J Crit Care Med. 2021;25(2):206-209. doi:10.5005/jpjournals-10071-23794
- Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, Patroniti N, Antonelli M, Pesenti A, Pappalardo F. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013 Sep;15(3):172-8. PMID: 23944202.