More Information
Submitted: June 29, 2023 | Approved: August 11, 2023 | Published: August 14, 2023
How to cite this article: Ssouni O, Ghannam A, El-Ahmadi B, Belkhadir Z, Abidi K, et al. The Predictive Value of Diaphragm Thickness Fraction on Postoperative Pulmonary Complications after Digestive Cancer Curative Surgery. Arch Surg Clin Res. 2023; 7: 035-045.
DOI: 10.29328/journal.ascr.1001072
Copyright License: © Ssouni O, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Postoperative pulmonary complications; Digestive cancer curative surgery; Diaphragm thickness fraction; Ultrasonography
The Predictive Value of Diaphragm Thickness Fraction on Postoperative Pulmonary Complications after Digestive Cancer Curative Surgery
Oussama Ssouni1*, Abdelilah Ghannam1, Brahim El-Ahmadi1, Zakaria Belkhadir1, Khalid Abidi2, Amal Bouziane3,4 and Redouane Abouqal4,5
1Department of intensive care, National Institute of Oncology, Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco
2Medical Intensive Care Unit, Ibn Sina Hospital, Mohammed V University in Rabat, Morocco
3Department of Periodontology, Faculty of Dental Medicine, Mohammed V University in Rabat, Morocco
4Laboratory of Biostatistics, Clinical Research, and Epidemiology, Mohammed V University in Rabat, Morocco
5Acute Medical Unit, Ibn Sina University Hospital, Rabat, Morocco
*Address for Correspondence: Oussama Ssouni, Department of intensive care, National Institute of Oncology, Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco, Email: [email protected]
Background: Postoperative Pulmonary Complications (PPCs) escalate mortality, hospitalization, and costs. This study aimed to predict PPCs after curative digestive cancer surgery using thickness fraction (TFdi) determined by ultrasonography.
Methods: A prospective study was conducted over a period of 9 months. Diaphragmatic ultrasound was performed pre-surgery and repeated postoperatively (within 24 hours of ICU admission, then day 3). Right and left hemidiaphragm thickness at end-expiration (TEE) and peak-inspiration (TPI) were measured using ultrasonography. The maximal diaphragm thickening fraction during inspiration (TFdi,max) was calculated: TFdi,max = (TPI–TEE)/TEE. Patients were classified into No-PPCs and PPCs groups.
Results: 159 patients participated, 55 (34.6%) developed PPCs. ICU stay was longer in PPCs patients with more deaths. TFdi,max decreased postoperatively and remained lower in PPCs patients [44.83% ± 11.07 vs. 31.54% ± 8.45; p < 0.001]. The receiver operating characteristic curve yielded an area under the curve of 0.83 [95% IC: 0.754 – 0.887]. TFdi,max < 37% had 72.7% sensitivity (95% IC: 59.0% – 83.8%) and 80.8% specificity (95% IC: 71.8% – 87.8%), Positive and negative Likelihood Ratios were 3.7 (95% IC: 2.4 – 5.7) and 0.3 (95% IC:0.2 – 0.5), respectively. In multiple logistic regression, preoperative risk factors for PPCs included TFdi,max < 37% [OR: 7.10; 95% CI: 1.71 – 18.60; p < 0.001] and supramesocolic surgery [OR: 9.94; 95% CI: 3.62 – 27.29; p < 0.001]. Epidural administration was protective [OR: 0.21; 95% CI: 0.052 – 0.87; p = 0.031].
Conclusion: A low preoperative TFdi,max identifies high-risk PPCs patients after digestive cancer surgery, aiding targeted preventive strategies like inspiratory muscle preoperative training.
Epidemiology
Postoperative Pulmonary Complications (PPCs) significantly escalate mortality and contribute to extended hospital stays, leading to increased costs [1]. The definition of PPCs has varied across literature, with the one proposed by Pedersen et al being a particularly suitable one. It encompasses any lung abnormality that results in identifiable lung disease or dysfunction, necessitating medical intervention, and adversely affecting a patient's clinical progression. This comprehensive definition encompasses atelectasis, lung infections, respiratory failure, prolonged mechanical ventilation, exacerbation of underlying sub-respiratory disease, and bronchospasm [2]. The incidence of PPCs in major surgery spans a range from <1% to 23% [3-10]. Notably, pulmonary complications surpass cardiac complications in prevalence [11-13], with postoperative respiratory failure being the most frequent PPC [10-14]. Patients with PPCs face increased short-term and long-term mortality, with a significant portion (14% - 30%) succumbing within 30 days post-major surgery compared to 0.2% - 3% without PPCs [6,7,9,15-17]. Long-term data from observational studies reveal substantial disparities in mortality rates between patients with and without PPCs: 45.9% vs. 8.7% at 1 year and 71.4% vs. 41.1% at 5 years [17]. PPCs also heighten morbidity, extending hospital stays by 13 - 17 days [9,11,18]. Instances such as postoperative respiratory failure necessitating unplanned re-intubation, typically within 72 hours of surgery [16,19], significantly amplify morbidity and length of hospital stay [16,18]. The occurrence of PPCs escalates healthcare costs primarily due to prolonged hospital stays [1].
Impact on surgery
A substantial majority of PPCs, about 85%, manifest within the initial three postoperative days [20]. This occurrence is partly attributed to early postoperative pathophysiological reductions in lung volumes [21], which, if prolonged, can lead to severe atelectasis, hypoxemia, and pneumonia [22,23]. The strength of respiratory muscles has been suggested to play a role in the development of certain PPCs [24,25]. Post-surgery, patients must cope with increased respiratory workload, reopen areas of lung atelectasis, and generate an effective cough to mobilize secretions. Interventions that fortify respiratory muscles before surgery and reduce their workload afterward have demonstrated benefits in randomized controlled clinical trials for PPC prevention [21,25-27]. The diaphragm, the primary respiratory muscle, can be assessed by measuring its thickness fraction (TFdi) at the attachment zone using ultrasound [28]. TFdi has shown reliability [29] and validation against trans-diaphragmatic pressure measurements [30], and is a strong predictor of successful mechanical ventilation weaning [31,32]. The potential of this diaphragmatic function marker was assessed by Cavayas and colleagues in cardiac surgery [33-35]. However, no prior study has explored the value of TFdi,max in a specific perioperative context, specifically digestive cancer curative surgery. Thus, our study aims to evaluate the diagnostic performance of preoperative ultrasound measurement of maximum TFdi for predicting PPCs in patients undergoing digestive cancer curative surgery.
Study design and settings
We conducted a prospective cohort study at the National Institute of Oncology in Rabat, Morocco. It is an academic center encompassing several specialties (medical oncology, ICU, radiotherapy, radiology, and digestive cancer surgery). The patients followed in this establishment are mainly affected by malignant diseases. The duration of the study was approximately nine months, between May 2019 and January 2020. Participants were recruited during the 48 hours before performing the surgery.
Participants
Patients 16 years of age or older undergoing digestive cancer curative surgery (laparotomy or laparoscopy) were eligible for inclusion in the study. The exclusion criteria were: urgent surgery, the existence of a pre-existing neuromuscular pathology, and the need for preoperative ventilatory support.
Diaphragm thickness fraction
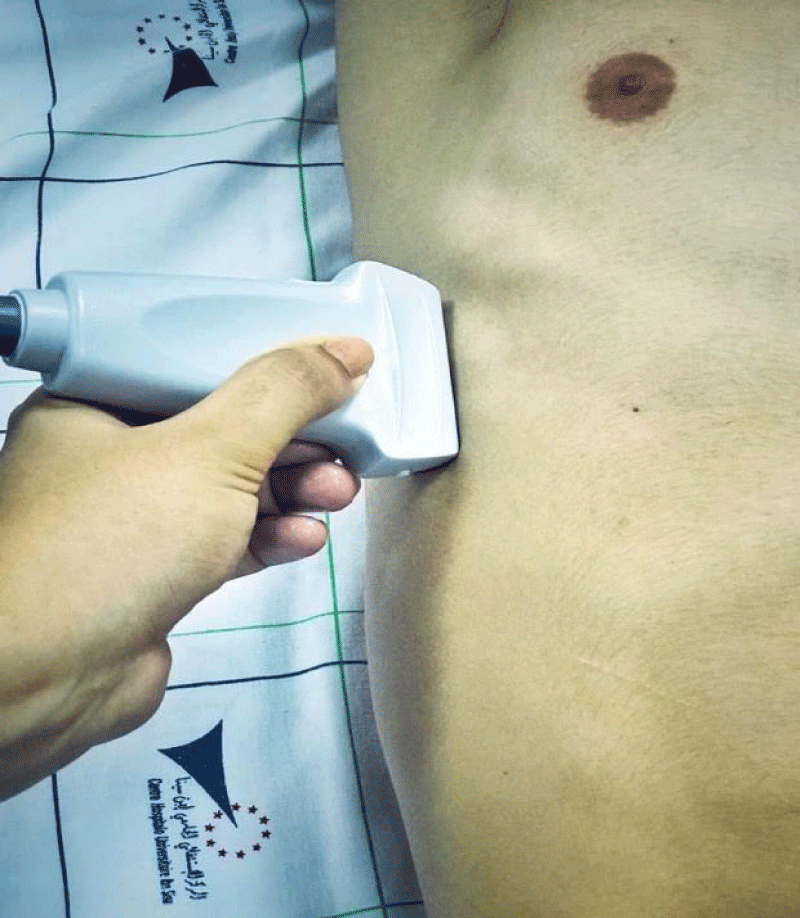
Diaphragmatic ultrasound was performed 24 to 48 hours before digestive cancer curative surgery, during the patient's hospitalization period in the surgical department, then repeated postoperatively (within 24 hours after his admission to intensive care, and on day 3). The patient was placed in a semi-seated position with the headboard at 45 degrees. Postoperative analgesia has been systematically optimized using a Visual Analog Scale (VAS), with the objective of a VAS <5 before performing ultrasound measurements. The TFdi measurement was carried out at the level of the Zone of Apposition (ZOA) of the right and left hemidiaphragm along the midaxillary line (Figures 1,2).
Figure 1: Probe placement to explore the diaphragm in the Zone of Apposition (ZOA).
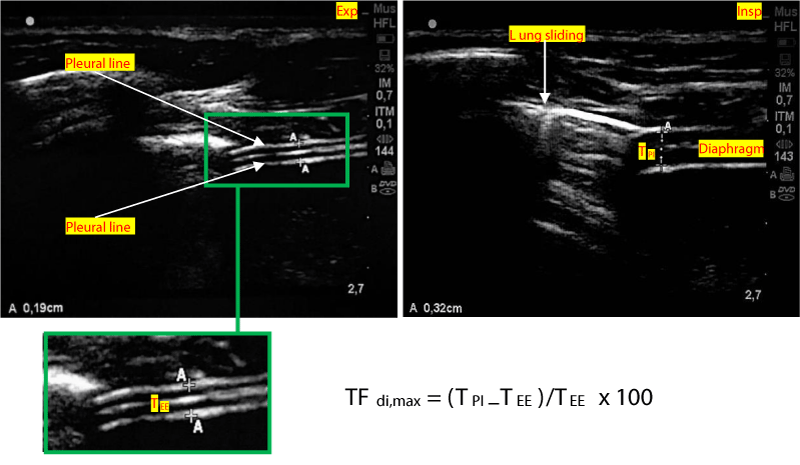
Figure 2: Method of measuring the diaphragm thickness fraction. TEE: Thickness at end-expiration. TPI: Thickness at end peak inspiration. TFdi,max = (TPI–TEE) / TEE x 100
A linear high-frequency probe (13 - 6 MHz) is placed along the mid-axillary line. The zone of apposition of the diaphragm with the chest wall is located caudal to the costodiaphragmatic recess. A good acoustic window is found between two ribs, and a 2D clip is acquired while the patient is asked to perform a maximal inspiratory effort, starting from functional residual capacity. The thickness of the diaphragm is measured at end-expiration (TEE) and peak-inspiration (TPI). TFdi,max is calculated with the following formula: TFdi,max = (TPI–TEE)/TEE. TFdi,max = maximal diaphragmatic thickness fraction during inspiration [36-39].
The TFdi was calculated at the time of data analysis using the following formula: TFdi,max = (TPI - TEE)/TEE [9]. TFdi was calculated for each hemidiaphragm, then an average value was assigned for each patient, which was used to reflect the overall diaphragmatic function. The treating team was not notified of the results of the examination.
Covariates
For each patient, we evaluated their age, gender, weight, height, body mass index (BMI), nutritional status, smoking, comorbidities, ASA Physical Status Classification [34], estimated functional capacity in metabolic equivalents (METs) was estimated from standard published tables based on protocol and total time completed in the final stage [35]. Also, we collected some biological data such as preoperative kalemia and albuminemia and pre and post-operative hemoglobin. We also collected information related to surgical and anesthetic procedures.
Anesthetic Procedure
The anesthesia protocol systematically included anesthesia balanced by propofol associated with fentanyl and non-depolarizing curare (rocuronium or cis-atracurium). Etomidate was proposed for patients with precarious hemodynamic conditions. The monitoring of the neuromuscular block was carried out by the analysis of the «train of four» at the adductor of the thumb. Locoregional analgesia by an epidural catheter or Rachianalgesia with morphine 0.2 mg was performed as much as possible. Multimodal analgesia included Paracetamol (except in cirrhotic patients) and morphine according to VAS was started postoperatively. Recourse to a morphine PCA could be proposed as soon as the patient was admitted to ICU. Extubation was carried out as early as possible. Decurarization was carried out systematically. The indication of post-operative supervision in the ICU was foreseen from the pre-operative period and depended on the patient's history and the type of surgery.
Outcomes
The principal endpoint of the study was the development of PPCs. This was a composite parameter defined by the occurrence of pneumonia, atelectasis, hypoxemia (PaO2 < 60 mmHg), respiratory acidosis (pH < 7.38), pleural effusion, rescues to non-invasive ventilation (NIV), reintubation, or the need for prolonged mechanical ventilation in the postoperative period. The patient's medical record was reviewed to determine if the diagnosis of PPCs was made by the treating team. Clinicians were not aware of any of the measured parameters in the study.
Definitions
Pneumonia was defined according to the Centers for Disease Control definition [40]. Atelectasis was defined as a new postoperative lung consolidation in the absence of pneumonia criteria on a chest x-ray. Hypoxemia and respiratory acidosis were defined by measurement of PaO2 < 60 mmHg and pH < 7.38 respectively. The diagnosis of pleural effusion was made by standard radiography or pleuropulmonary ultrasound. Mechanical ventilation was defined as prolonged if it lasted more than 24 hours after the operation, as defined by the Society of Thoracic Surgeons (STS) [41].
Statistical analyses
Data are presented as the mean ± standard deviation for variables with a normal distribution and as the median and interquartile range for variables with skewed distribution. Categorical variables were presented as counts and percentages. Parametric or nonparametric tests were used for continuous variables as appropriate after the normality of the distribution was tested by the Kolmogorov-Smirnov test. Group comparisons of continuous variables were made using the Student t-test or Mann-Whitney U test, as appropriate. For the categorical variables, group comparisons used the chi-2 test or the exact Fischer test. Analysis of variance (ANOVA) for repeated measures was used to compare continuous variables over time. Bonferroni's post hoc test was used to locate the significance. Receiver operating characteristic curves and the respective areas under the curves (AIC) were calculated for TFdi,max. The best cutoff value was chosen using Youden's index. The sensitivity, specificity, and positive and negative likelihood ratios (LH) (with 95% confidence intervals (95% CI)) were calculated at the best cutoff value. This value was used to divide patients into two categories. Univariate and multiple logistic regressions were performed to identify factors potentially associated with the occurrence of PPCs. Variables with a value of p < 0.1 in univariate analyses were introduced to the multiple models. Other variables known in the PPCs literature were forced into the multiple models. Results are presented as the odds ratio (OR) and 95% CI. A two-tailed p value <0.05 was considered significant. Assuming a 30% occurrence of PPC [42], a sample size of 160 patients was required to detect an OR 2.5 for a low TFdi,max with 80% power and a two-sided alpha of 0.05. The analyses were conducted with IBM SPSS Statistics (IBM Corp, Armonk, NY, USA).
Ethical considerations
The study was carried out in accordance with the Helsinki Declaration. Our protocol was approved by the Committee on Ethics for Biomedical Research of the Faculty of Medicine and Pharmacy of Rabat. Free and informed consent was obtained.
Patient characteristics
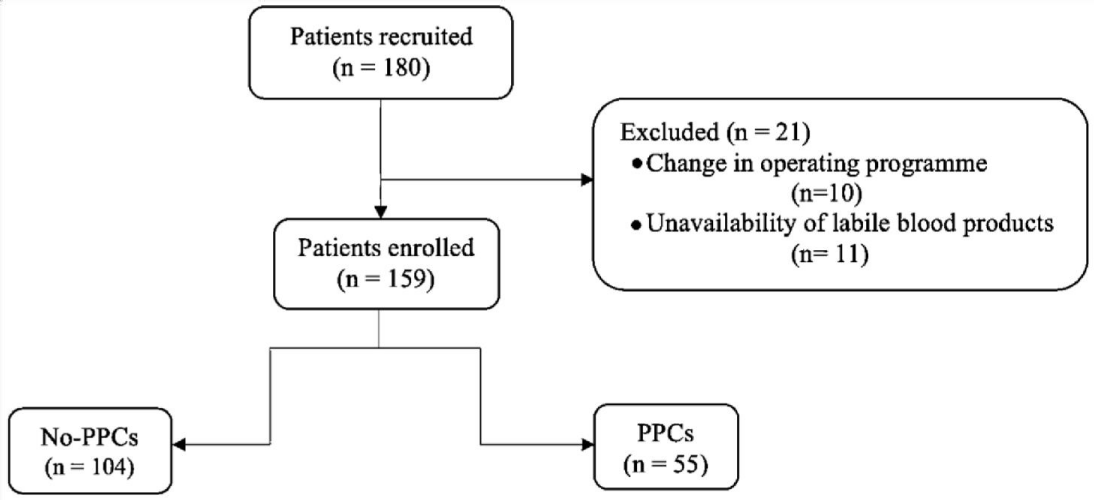
During the study period, 238 patients were admitted to ICU. 180 patients were recruited, of which 159 were included for analysis. 21 patients were excluded after registration for problems with the management of the surgical program or because of the unavailability of labile blood products on the day of surgery (Figure 3). Demographic and clinical characteristics of the study are shown in Table 1. The age of the study population was 55.5 ± 8.1 years, and 52.8% were male. In our study, ICU mortality was 7.5% (12 patients died).
Figure 3: Study flow chart.
| Table 1: Sample characteristics. | ||||
| Parameter | Total (n = 159) | No-PPCs (n = 104) | PPCs (n = 55) | p - value |
| Age (years)a | 55.5 ± 8.16 | 54.98 ± 9.21 | 56.71 ± 5.57 | 0.14 |
| Gender (n (%)) | 1 | |||
| Men | 84 (52.8) | 55 (52.9) | 29 (52.7) | |
| Women | 75 (47.2) | 49 (47.1) | 26 (47.3) | |
| Weight (kg)a | 63.21 ± 8.80 | 62.82 ± 8.54 | 64 ± 9.31 | 0.43 |
| Cut (m)a | 1.64 ± 0.04 | 1.64 ± 0.04 | 1.64 ± 0.04 | 0.73 |
| BMI (Kg/m2)a | 23.6 ± 3.94 | 23.40 ± 3.88 | 23.9 ± 4.07 | 0.45 |
| Undernutrition (n (%)) | 17 (10.7) | 7 (6.7) | 10 (18.2) | 0.026 |
| OSAS (n (%)) | 16 (10.1) | 12 (11.5) | 4 (7.3) | 0.39 |
| ASA (n (%)) | 0.16 | |||
| I | 77 (48.4) | 49 (47.1) | 28 (50.9) | |
| II | 67 (42.1) | 48 (46.2) | 19 (34.5) | |
| III | 15 (6.9) | 7 (6.7) | 8 (14.5) | |
| Functional capacity (n (%)) | 0.067 | |||
| > 4MET | 116 (73) | 71 (68.3) | 45 (81.8) | |
| < 4MET | 43 (27) | 33 (31.7) | 10 (18.2) | |
| Smoking (n (%)) | 0.54 | |||
| Non-smoker | 98 (61.6) | 62 (59.6) | 36 (65.5) | |
| Ex-smoker | 37 (23.3) | 27 (26) | 10 (18.2) | |
| Smoker | 24 (15.1) | 15 (14.4) | 9 (16.4) | |
| COPD (n (%)) | 10 (6.3) | 7 (6.7) | 3 (5.5) | 0.96 |
| Heart disease (n (%)) | 22 (13.8) | 14 (13.5) | 8 (14.5) | 0.85 |
| CRF (n (%)) | 4 (2.5) | 2(1.9) | 2(3.6) | 0.60 |
| Arterial hypertension (n (%)) | 53 (33.3) | 34 (32.7) | 19 (34.5) | 0.81 |
| Diabetes (n (%)) | 42 (26.4) | 28 (26.9) | 14 (25.5) | 0.84 |
| Preoperative Hb (g/dl)a | 12.10 ± 1.46 | 12.37 ± 1.45 | 11.6 ± 1.37 | 0.001 |
| Postoperative Hb (g/dl)a | 10.68 ± 1.24 | 10.81 ± 1.25 | 10.44 ± 1.21 | 0.07 |
| Kalemia (mmol/L)a | 4.2 ± 0.62 | 4.17 ± 0.61 | 4.25 ± 0.64 | 0.44 |
| Albuminemia (g/L) | 32.36 ± 3.14 | 32.70 ± 3.18 | 31.73 ± 2.99 | 0.06 |
| Type of surgery (n (%)) | <0.001 | |||
| Upper abdominal surgery | 48 (30.2) | 19 (18,3) | 29 (52.7) | |
| Submesocolic | 111 (69.8) | 85 (81.7) | 26 (47.3) | |
| Laparoscopy (n (%)) | 83 (52.2) | 57 (54.8) | 26 (47.3) | 0.36 |
| Surgical duration (hours)a | 4.49 ± 0.87 | 4.42 ± 0.80 | 4.62 ± 0.98 | 0.17 |
| Intraoperative transfusion (n (%)) | 20 (12.6) | 14 (13.5) | 6 (10.9) | 0.64 |
| Epidural analgesia (n (%)) | 27 (17) | 23 (22.1) | 4(7.3) | 0.018 |
| Rachianalgesia (n (%)) | 88 (55.3) | 62 (59.6) | 26 (47.3) | 0.13 |
| Postoperative morphine (n (%)) | 115 (72.3) | 71 (68.3) | 44 (80) | 0.11 |
| Extubation delay (min)b | 90 (60-120) | 90 (45-90) | 90 (60-120) | 0.37 |
| LOS in ICU (days)b | 4 (1-7) | 1 (1-5) | 7 (6-9) | <0.001 |
| ICU mortality (n (%)) | 12 (7.5) | 4 (3.8) | 8 (14.5) | 0.024 |
| a expressed as mean ± standard deviation, b expressed as median (interquartile range). BMI: Body Mass Index; OSAS: Obstructive Sleep Apnea Syndrome; ASA: American Society of Anesthesiologists; MET: Metabolic Equivalent; COPD: Chronic Obstructive Pulmonary Disease; CRF: Chronic Respiratory Failure; Hb: Hemoglobin; LOS: Length of Sta;, ICU: Intensive Care Unit. | ||||
Postoperative pulmonary complications
Overall, 55 patients (34.6%) developed the composite PPCs result. 28 patients developed atelectasis (17.6%), 27 patients (17%) used NIV postoperatively, hypoxemia was found in 24 patients (15.1%), 10 patients were reintubated (6.3%) and 8 patients developed pneumonia (5%) (Table 2).
| Table 2: Comparison of occurrence of PPCs depending on survivors and the dead. | ||||
| Variables (n (%)) | Total (n =159) | Survivors (n =147) | Dead (n = 12) | p - value |
| Atelectasis | 28 (17.6) | 25 (17) | 3 (25) | 0.44 |
| Hypoxemia | 24 (15.1) | 19 (12.9) | 5 (41.7) | 0.020 |
| Pneumonia | 8 (5) | 3 (2) | 5 (41.7) | <0.001 |
| Respiratory acidosis | 7 (4.4) | 6 (4.1) | 1 (8.3) | 0.42 |
| Recourse to the NIV | 27 (17) | 26 (17.7) | 1 (8.3) | 0.69 |
| Use of oxygen | 36 (22.6) | 30 (20.4) | 6 (50) | 0.029 |
| Pleural effusion | 5 (3.1) | 4 (2.7) | 1 (8.3) | 0.32 |
| Extubation failure | 10 (6.3) | 6 (4.1) | 4 (33.3) | 0.003 |
| PPCs (composite) | 55 (34.6) | 47 (32) | 8 (66.9) | 0.024 |
| Expressed in effective (percentage). NIV: Non-Invasive Ventilation; PPCs: Postoperative Pulmonary Complications. | ||||
Patients with PPCs were more undernourished [7 (6.7) vs. 10 (18.2); p = 0.026], and had a lower pre-operative hemoglobin [11.6 ± 1.37 vs. 12.37 ± 1.45; p = 0.001]. In terms of anesthetic and surgical techniques, supramesocolic surgery was more often complicated by PPCs [29 (52.7) vs. 26 (47.3); p < 0.001], whereas epidural patients developed significantly fewer PPCs [23 (22.1) vs. 4(7.3); p = 0.018]. The length of ICU stays was significantly longer in patients with PPCs [4 (1-7) vs. 1 (1-5) days, p < 0.001]. In terms of mortality, it was significantly higher in the PPCs group [4 (3.8) vs. 8 (14.5); p = 0.024].
Diaphragmatic ultrasound
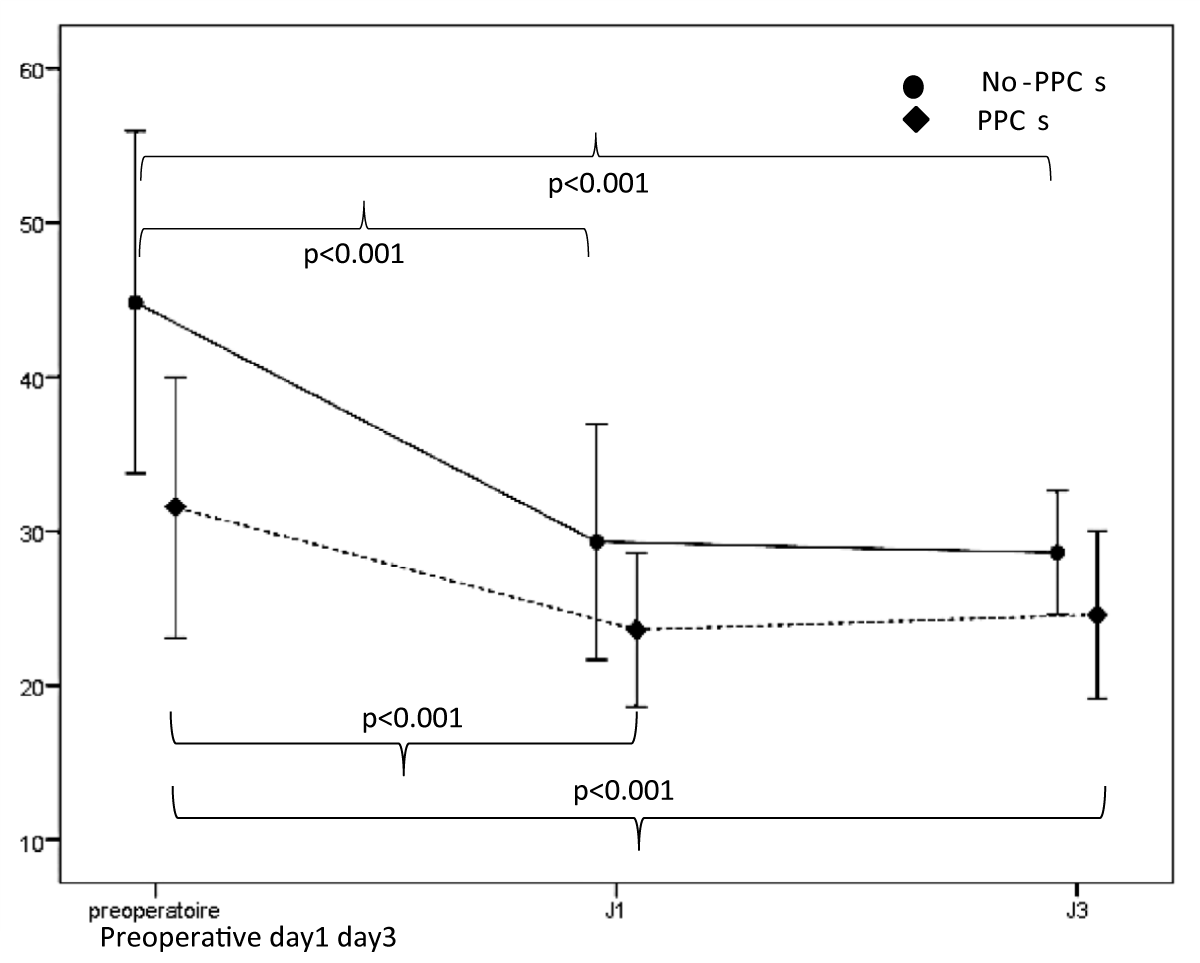
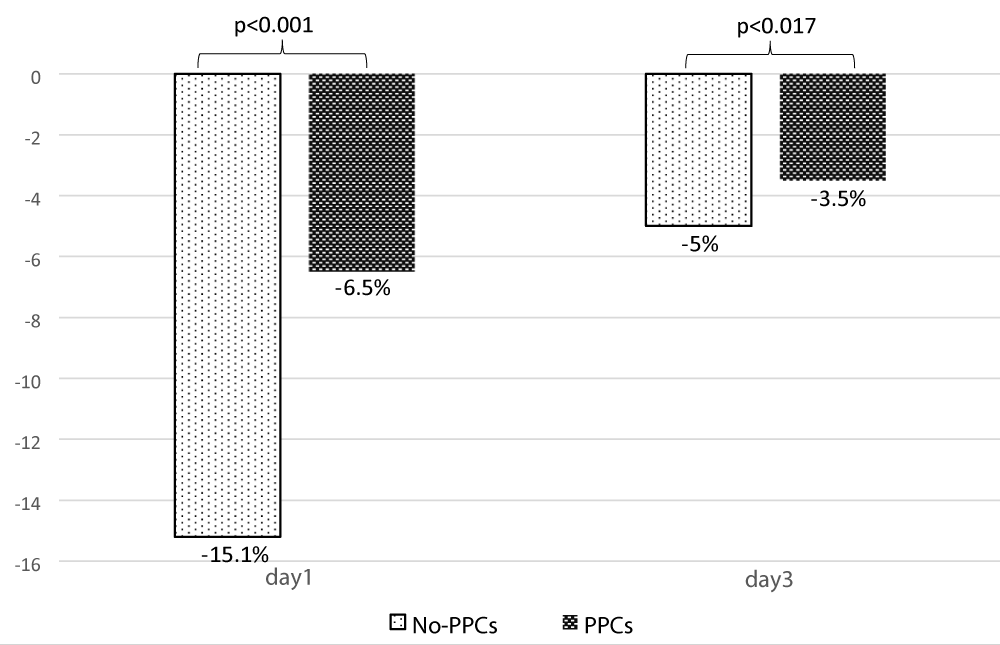
Regarding the analysis of ultrasound data, the mean TFdi,max was 40.23% ± 12.02, It was significantly less in patients with PPCs [44.83% ± 11.07 vs. 31.54% ± 8.45; p < 0.001] (Table 3). Its value decreased significantly on day 1 and day 3 compared to its preoperative value (Figure 4). The postoperative change in TFdi,max observed in our study was significantly greater in patients without PPCs [-15.2% (-22, -9) vs. -6.5% (-9.5, -4.5) p < 0.001] (Figure 5).
| Table 3: Ultrasound Data | ||||
| Parameter | Total (n = 159) | No-PPCs (n = 104) | PPCs (n = 55) | p - value |
| TFdi,max preoperative(%)a | 40.23 ± 12.02 | 44.83 ± 11.07 | 31.54 ± 8.45 | <0.001 |
| TFdi,max day(%)a | 27.34 ± 7.34 | 29.31 ± 7.63 | 23.61 ± 4.98 | <0.001 |
| TFdi,max day3(%)a | 26.46 ± 5.19 | 28.61 ± 3.98 | 24.59 ± 5.43 | <0.001 |
| Change of TFdi,max at day1(%)b | -10 ( -21,-6,5) | -15.2 (-22, -9) | -6.5 (-9.5, -4.5) | <0.001 |
| Change of TFdi,max at day3 (%)b | -5 (-6,5, -3) | -5 (-6.5, -3.2) | -3.5 (-6, -2.5) | 0.017 |
| a expressed as mean standard deviation, b expressed as median (interquartile interval), TFdi,max: maximum diaphragm thickness fraction. | ||||
Figure 4: Evolution of TFdi,max between its preoperative value, day 1 and day 3.
Figure 5: Change in TFdi,max between preoperative and day1, preoperative and day 3.
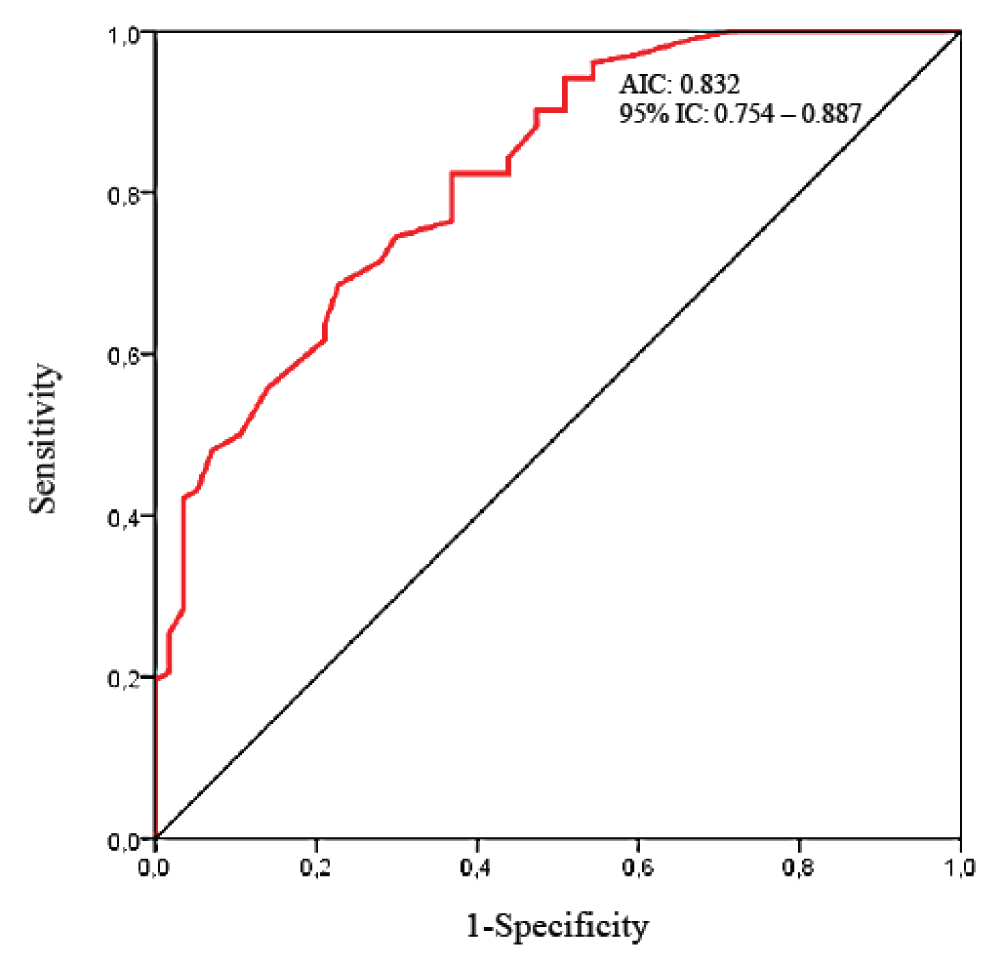
Predictive value of diaphragmatic thickness fraction The performance of diaphragmatic ultrasound to predict a patient at risk of PPCs is summarized in Table 4 and the ROC curve is shown in Figure 6. The best predictor was the presence of a preoperative TFdi,max < 37% [Sensitivity: 72.7%, 95% IC: 59.0 – 83.8; Specificity: 80.8, 95% IC: 71.8 – 87.8; PPV: 66.6%; NVP: 84.8%; AIC: 0.832, 95% IC: 0.754 – 0.887]. Positive and negative Likelihood Ratio were respectively 3.7 (95% IC: 2.4 – 5.7) and 0.3 (95% IC:0.2 – 0.5), which is a moderate value of discrimination between the PPCs and no-PPCs group.
| Table 4: Diaphragmatic ultrasound performance to predict the risk of PPCs occurring at a TFdi. | ||
| Cutoff value: TFdi = 37% | Values | 95% IC |
| Sensitivity | 72.7% | 59.0 – 83.8 |
| Specificity | 80.8% | 71.8 – 87.8 |
| Positive Likelihood Ratio | 3.7 | 2.4 – 5.7 |
| Negative Likelihood Ratio | 0.3 | 0.2 – 0.5 |
| Prevalence of the disease | 34.5% | 27.2 – 42.5 |
| Positive Predictive Value (PPV) | 66.6% | 56.6 – 75.3 |
| Negative predictive value (NPV) | 84.8% | 78.2 – 89.7 |
| Accuracy | 77.9% | 70.7 – 84.1 |
Figure 6: Receiver Operating Curve (ROC) of preoperative TFdi,max for discrimination of No-PPCs and PPCs groups after digestive cancer curative surgery.
Diaphragmatic thickness fraction predictors of PPCs
In multiple logistic regression, the TFdi,max was included in the regression model. TFdi,max < 37% [OR: 7.10; 95% CI: 1.71 – 18.60; p < 0.001] and supramesocolic surgery [OR: 9.94; 95% CI: 3.62 – 27.29; p < 0.001] were independently related to the occurrence of PPCs. However, performing an epidural was a protective factor [OR: 0.21; 95% CI: 0.052 – 0.87; p = 0.031] (Table 5).
| Table 5: Preoperative risk factors of PPCs. | ||||||
| Variable |
Univariate analysis | Multivariate analysis | ||||
| OR | 95% IC | p | OR | 95% IC | p | |
| Age | 1.02 | 0.98 – 1.07 | 0.20 | 1.01 | 0.94 – 1.07 | 0.76 |
| BMI | 1.03 | 0.95 – 1.12 | 0.44 | – | – | – |
| Undernutrition | 3.08 | 1.10 – 8.61 | 0.032 | 1.01 | 0.94 – 1.07 | 0.75 |
| ASA | – | – | – | |||
| I | 1 | |||||
| II | 0.69 | 0.34 – 1.40 | 0.30 | |||
| III | 2 | 0.65 – 6.10 | 0.22 | |||
| Smoking | – | – | – | |||
| Non-smoker | 1 | |||||
| Ex-smoker | 0.63 | 0.27 – 1.46 | 0.94 | |||
| Smoker | 1.03 | 0.41 – 2.6 | 0.29 | |||
| COPD | 1.25 | 0.31 – 5.04 | 0.75 | – | – | – |
| Functional capacity | – | – | – | |||
| > 4MET | 1 | |||||
| < 4MET | 2.09 | 0.94 – 4.65 | 0.15 | |||
| Preoperative Hb | 0.67 | 0.52 – 0.86 | 0.002 | 0.84 | 0.61 – 1.16 | 0.29 |
| Epidural Type of surgery |
0.018 |
0.10 – 0.95 |
0.041 |
0.21 |
0.052 – 0.87 |
0.031 |
| Submesocolic | 1 | |||||
| Upper abdominal surgery | 5 | 2.41 – 10.31 | <0.001 | 9.94 | 3.62 – 27.29 | <0.001 |
| TFdi,max <37% | 8 | 3.81 – 16.78 | <0.001 | 7.10 | 1.71 – 18.60 | <0.001 |
| BMI: Body Mass Inde;, ASA: American Society of Anesthesiologists, MET: Metabolic Equivalen;, COPD: Chronic Obstructive Pulmonary Diseas;, Hb: Hemoglobi;, TFdi,max: maximum diaphragm thickness fraction. | ||||||
In our prospective cohort of digestive cancer curative surgery, 55 patients (34.6%) of the 159 included in the study developed PPCs. Supramesocolic surgery was the main risk factor associated with their occurrence. However, the introduction of epidural analgesia was a protective factor. The analysis of the ROC curve showed that a pre-operative TFdi,max of less than 37% was associated with the occurrence of PPCs with a sensitivity of 72.7% and a specificity of 80.8%. TFdi,max decreased postoperatively and remained lower in patients with PPCs. In terms of "outcome", the length of stay in the ICU was significantly longer in patients who developed PPCs with significantly more deaths.
PPCs are as common as cardiovascular complications. Their incidence varies greatly in the literature. This is due to the multiple definitions that have been used. Moreover, few studies have studied the occurrence of these PPCs in a homogeneous population taking into account the surgical technique, the path first, the existence of an induction treatment, and so on. However, the percentage between 10% and 25% according to the series [43-46] seems to be currently falling. In general, and according to standardized definitions, the rate of pulmonary complications is 39% and they account for nearly 60% of hospital deaths. In our cohort, the PPCs rate was around 34.6%, with significantly higher mortality.
The factors favoring the occurrence of PPCs are multiple [43-53]. Some are linked to the patient (poor pre-operative ventilator performance, smoking, nutritional status, induction treatment). Others are related to the intervention (duration of the operation, mechanical ventilation, transthoracic surgical approach). Finally, the postoperative period, in particular the management of pain and fluid intake, also seems to be involved in the genesis of these complications. The site of the incision is an important predictor of PPCs. The ventilatory impact of supraumbilical digestive surgery is a risk factor regardless of patient history. The risk decreases when the incision moves away from the diaphragm [54-56].
Our results corroborate this effect, in our multiple regression model, upper abdominal surgery was identified as a risk factor independently related to the occurrence of PPCs [OR: 9.94; 95% CI: 3.62 – 27.29; p < 0.001].
These «macro-events» probably mask the protective influence that can have some practices, perfectly known from high volume centers: protocolized management of perfused volumes in intraoperative, early extubation, choice of epidural analgesia for the postoperative period, noninvasive ventilation.
The thoracic epidural analgesia has several theoretical elements suggesting a decrease in pulmonary complications of heavy surgery, especially major abdominal ones. A complete and didactic review of the literature has been published [57]. Epidural analgesia with local anesthetics alone or, better, in combination with opiates, appears to be more effective on pain than parenteral opioid analgesia, including self-administered administration. Therefore, it should promote coughing and early postoperative mobilization. In addition, by blocking the inhibitory reflexes of diaphragmatic function seen in abdominal surgery, epidural analgesia with local anesthetics is expected to improve diaphragmatic dysfunction. Evidence of a beneficial effect on gastric graft vitality and PPCs has recently been provided [58]. Again, our study showed that the use of an epidural for postoperative analgesia was associated with a reduction in PPCs [OR: 0.21; 95% CI: 0.052 – 0.87; p = 0.031].
A relative weakness of the diaphragm was very common in the patients included in the study. The mean value of TFdi in healthy subjects is approximately 80% - 100% with the 5th percentile of 20% - 30% [59]. In comparison, the pre-operative TFdi mean for patients in our study was 40% or half of normal. One in four patients had TFdi below the fifth percentile of normal. Several factors may explain why the preoperative diaphragmatic contractile reserve of our patients is altered. The neoplastic context of our population explains part of this result [60,61]. The current treatment of digestive cancers is part of multidisciplinary management based on neoadjuvant Radio-Chemotherapy (RCT). Nearly 50% of patients undergoing surgery are currently benefiting from this strategy. The presence of induction therapy is considered by some teams [43,57,58] as a factor of hospital excess mortality but the controversy continues as many studies have not found an obvious relationship between the occurrence of PPCs and RCT [62]. Radiation therapy, depending on the doses administered, adds the possibility of lung or pleural parenchymal involvement and promotes surgical complications. The PPCs rates depend closely on the volume of irradiation. The higher the dose of radiation therapy, the greater the incidence and severity of PPCs. The same applies to the diffusion capacities of the alveolar-capillary membrane [62]. Chemotherapy participates in general immunosuppression and promotes postoperative infectious events. As a result, unexpected bacterial or viral agents were identified on postoperative lung biopsies, particularly after RCT [63].
In addition, cancer and chemotherapy are major risk factors for undernutrition. In our cohort, the presence of preoperative undernutrition was significantly associated with the occurrence of PPCs [OR: 3.08; 95% CI: 1.10 – 8.61; p = 0.32]. Undernourished patients with or without chronic obstructive pulmonary disease have atrophy of the accessory respiratory muscles and diaphragm [64] with decreased maximum ventilation per minute [65]. Malnutrition also leads to decreased ventilatory responses to hypoxia and hypercapnia. The occurrence of atelectasis is theoretically favored by undernutrition. Indeed, it is experimentally demonstrated that undernutrition decreases surfactant synthesis. On the other hand, there is a decrease in the frequency of sighs and a decrease in the strength of the expiratory muscles [65]. The increased risk of infection, particularly in the lung of undernourished patients, is a classic notion. The occurrence of pneumopathy following digestive surgery is more common in patients with preoperative protein malnutrition.
Decreased diaphragmatic function in postoperative is almost constant after major abdominal surgery. The occurrence of PPCs is the combination of deleterious effects of anesthesia, mechanical ventilation, and surgery on respiratory function. General anesthesia modifies ventilation/perfusion ratios, as well as the value of chest volumes and compliances. This results from the combination of supine position and muscle relaxation. It is the consequence of ventilatory disorders or atelectasis present mainly in declivized areas. These phenomena are exacerbated by a diaphragmatic dysfunction related to a reflex inhibition of phrenic innervation, presumably not related to pain since the administration of morphine by the epidural route does not improve the diaphragmatic function. Only the use of local anesthetics as part of epidural analgesia can lead to an improvement in diaphragmatic dysfunction, which however remains only partial [66]. Diaphragmatic atony will persist for several hours or even several days, either by neurogenic reaction or by direct trauma related to surgery (stretching, compression, phrenotomy). These combined factors constitute a restrictive syndrome that persists for more than two weeks but is particularly important during the first seven days. All these elements are added to the digestive distension relative to the postoperative ileus to reduce the effectiveness of the cough.
In addition, the adverse effects of mechanical ventilation have been incriminated in recent years in the genesis of PPCs. It has been shown that a few hours of fully controlled mechanical ventilation leads quickly to atrophy and a decrease in contractility strength both in vivo and in vitro [67-70]. Indeed, it seems that mechanical ventilation is capable of producing per se complications of mechanical, hydrostatic, and inflammatory origin called «lesions induced by mechanical ventilation» (Ventilator-Induced Lung Injury VILI) [71]. These lesions are attributed to barotrauma, volotrauma, and more recently biotrauma. These inflammatory phenomena could also continue with the maintenance of postoperative ventilation. The current recommendations recommend that the postoperative ventilation time be shortened to a minimum and that anesthetic protocols be used to allow for rapid extubation of the "fast track recovery" type [47,72]. Finally, some insist on the need for intraoperative corticosteroids to reduce the occurrence of inflammatory phenomena. However, the results of such practices remain insufficient to propose them systematically [73,74].
The ventilatory effects of upper abdominal surgery are mainly due to a decrease in diaphragmatic inspiratory function. This diaphragmatic dysfunction may be responsible for a 30% - 40% decrease in lung volumes in upper abdominal surgery [75]. These abnormalities are maximum on the first postoperative day but usually persist for two weeks [75]. This alteration of contractile function is multifactorial: surgical aggression, inflammatory reaction, anesthetic agents, and postoperative pain [76]. The mechanical aggression represented by parietal muscular decay during surgery is one of the main factors at the origin of postoperative diaphragmatic muscular dysfunction. However, the importance of parietal decay (and especially abdominal muscle sections) is not sufficient to explain the occurrence of diaphragmatic dysfunction, as it is also observed after laparoscopic surgery [77]. One of the main mechanisms of diaphragmatic dysfunction could be a reflex inhibition of the phrenic inspiratory discharge, the starting point of which would be at the level of the visceral afferences of the mesenteric region [78].
However, the postoperative change in TFdi observed in our study was significantly greater in patients without PPCs, this can be explained by a higher preoperative value of TFdi,max in patients without PPCs. Thus, it would appear that the preoperative TFdi,max value is the main predictor of PPCs occurrence. A low preoperative TFdi,max helped to predict the occurrence of CCPs after digestive cancer curative surgery. In contrast with previously described risk factors, diaphragm function could be improved with inspiratory muscle training with potentially positive impacts on outcomes.
Our cohort identified and described a simple, easy, and affordable means of inspirational muscle weakness that could help identify vulnerable patients who could benefit from preventive strategies. To our knowledge, no study has yet highlighted the contribution of diaphragmatic ultrasound in digestive cancer curative surgery to assess the risk of PPCs. However, our work has its limits. Our study did not analyze the impact of fragility and sarcopenia indicators in the genesis of PPCs. Also, skin marking was not used, making it possible to compare repeated measurements in the same patient at different times. Finally, Postoperative TFdi measurements may have been affected by a decrease in inspiratory effort in the context of postoperative pain despite resorting to a multimodal postoperative analgesia strategy.
However, despite a moderate discrimination value reflected by LR, it seems that the association between a TFdi,max less than 37%, and the occurrence of PPCs is strong. It is important to note that none of the limitations mentioned should have affected the relationship between preoperative mean TFdi,max, and the incidence of PPCs, which was our main analysis.
In this prospective cohort study conducted in adults undergoing digestive cancer curative surgery, we have demonstrated that a maximum average thickness fraction of less than 37% is a major predictor of PPCs, with a sensitivity of 72.7% and specificity of 80.8%. TFdi decreased significantly in the postoperative period and remained lower in patients with pulmonary complications. This easily accessible marker of diaphragmatic weakness could, therefore, help identify vulnerable patients who would benefit most from preventive strategies such as preoperative training of the inspiratory muscles. However, the 37% threshold value calculated in this preliminary study will need to be validated in future clinical studies that include a significant number of patients before it can be proposed for clinical practice.
Authors’ contributions: All authors contributed equally to the conceptualization and writing of the manuscript. The authors read and approved the final manuscript.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
- Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017 Mar 1;118(3):317-334. doi: 10.1093/bja/aex002. PMID: 28186222.
- Smetana GW, Lawrence VA, Cornell JE; American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006 Apr 18;144(8):581-95. doi: 10.7326/0003-4819-144-8-200604180-00009. PMID: 16618956.
- Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2009 Jun;110(6):1253-60. doi: 10.1097/ALN.0b013e31819faa71. PMID: 19417617.
- Bablekos GD, Michaelides SA, Analitis A, Charalabopoulos KA. Effects of laparoscopic cholecystectomy on lung function: a systematic review. World J Gastroenterol. 2014 Dec 14;20(46):17603-17. doi: 10.3748/wjg.v20.i46.17603. PMID: 25516676; PMCID: PMC4265623.
- Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res. 2015 Oct;198(2):441-9. doi: 10.1016/j.jss.2015.03.028. Epub 2015 Mar 18. PMID: 25930169.
- Kor DJ, Warner DO, Alsara A, Fernández-Pérez ER, Malinchoc M, Kashyap R, Li G, Gajic O. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011 Jul;115(1):117-28. doi: 10.1097/ALN.0b013e31821b5839. PMID: 21694510; PMCID: PMC3986041.
- McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005 Mar 1;171(5):514-7. doi: 10.1164/rccm.200408-1069OC. Epub 2004 Nov 24. PMID: 15563632.
- Fisher BW, Majumdar SR, McAlister FA. Predicting pulmonary complications after nonthoracic surgery: a systematic review of blinded studies. Am J Med. 2002 Feb 15;112(3):219-25. doi: 10.1016/s0002-9343(01)01082-8. PMID: 11893349.
- Smith PR, Baig MA, Brito V, Bader F, Bergman MI, Alfonso A. Postoperative pulmonary complications after laparotomy. Respiration. 2010;80(4):269-74. doi: 10.1159/000253881. Epub 2009 Oct 28. PMID: 19864881.
- Canet J, Sabaté S, Mazo V, Gallart L, de Abreu MG, Belda J, Langeron O, Hoeft A, Pelosi P; PERISCOPE group. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: A prospective, observational study. Eur J Anaesthesiol. 2015 Jul;32(7):458-70. doi: 10.1097/EJA.0000000000000223. PMID: 26020123.
- Lawrence VA, Hilsenbeck SG, Mulrow CD, Dhanda R, Sapp J, Page CP. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995 Dec;10(12):671-8. doi: 10.1007/BF02602761. PMID: 8770719.
- Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med. 2006 Feb;21(2):177-80. doi: 10.1111/j.1525-1497.2006.00319.x. PMID: 16606377; PMCID: PMC1484655.
- Lawrence VA, Hilsenbeck SG, Noveck H, Poses RM, Carson JL. Medical complications and outcomes after hip fracture repair. Arch Intern Med. 2002 Oct 14;162(18):2053-7. doi: 10.1001/archinte.162.18.2053. PMID: 12374513.
- Mazo V, Sabaté S, Canet J, Gallart L, de Abreu MG, Belda J, Langeron O, Hoeft A, Pelosi P. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014 Aug;121(2):219-31. doi: 10.1097/ALN.0000000000000334. PMID: 24901240.
- Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000 Apr;92(4):977-84. doi: 10.1097/00000542-200004000-00014. PMID: 10754616.
- Ramachandran SK, Nafiu OO, Ghaferi A, Tremper KK, Shanks A, Kheterpal S. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology. 2011 Jul;115(1):44-53. doi: 10.1097/ALN.0b013e31821cf6de. PMID: 21552116.
- Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ; Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005 Sep;242(3):326-41; discussion 341-3. doi: 10.1097/01.sla.0000179621.33268.83. PMID: 16135919; PMCID: PMC1357741.
- Nafiu OO, Ramachandran SK, Ackwerh R, Tremper KK, Campbell DA Jr, Stanley JC. Factors associated with and consequences of unplanned post-operative intubation in elderly vascular and general surgery patients. Eur J Anaesthesiol. 2011 Mar;28(3):220-4. doi: 10.1097/EJA.0b013e328342659c. PMID: 21191304.
- Grosse-Sundrup M, Henneman JP, Sandberg WS, Bateman BT, Uribe JV, Nguyen NT, Ehrenfeld JM, Martinez EA, Kurth T, Eikermann M. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ. 2012 Oct 15;345:e6329. doi: 10.1136/bmj.e6329. PMID: 23077290; PMCID: PMC3473088.
- Serpa Neto A, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, Hollmann MW, Jaber S, Kozian A, Licker M, Lin WQ, Moine P, Scavonetto F, Schilling T, Selmo G, Severgnini P, Sprung J, Treschan T, Unzueta C, Weingarten TN, Wolthuis EK, Wrigge H, Gama de Abreu M, Pelosi P, Schultz MJ; PROVE Network investigators. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014 Dec;2(12):1007-15. doi: 10.1016/S2213-2600(14)70228-0. Epub 2014 Nov 13. Erratum in: Lancet Respir Med. 2014 Dec;2(12):e23. PMID: 25466352.
- Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000 May;92(5):1467-72. doi: 10.1097/00000542-200005000-00037. PMID: 10781293.
- Jaber S, Chanques G, Jung B. Postoperative noninvasive ventilation. Anesthesiology. 2010 Feb;112(2):453-61. doi: 10.1097/ALN.0b013e3181c5e5f2. PMID: 20068454.
- van Kaam AH, Lachmann RA, Herting E, De Jaegere A, van Iwaarden F, Noorduyn LA, Kok JH, Haitsma JJ, Lachmann B. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004 May 1;169(9):1046-53. doi: 10.1164/rccm.200312-1779OC. Epub 2004 Feb 20. PMID: 14977624.
- Hulzebos EH, Smit Y, Helders PP, van Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database Syst Rev. 2012 Nov 14;11(11):CD010118. doi: 10.1002/14651858.CD010118.pub2. PMID: 23152283; PMCID: PMC8101691.
- Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006 Oct 18;296(15):1851-7. doi: 10.1001/jama.296.15.1851. PMID: 17047215.
- García-Delgado M, Navarrete-Sánchez I, Colmenero M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol. 2014 Apr;27(2):146-52. doi: 10.1097/ACO.0000000000000059. PMID: 24514031.
- Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen-Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest. 2009 May;135(5):1252-1259. doi: 10.1378/chest.08-1602. Epub 2008 Nov 18. PMID: 19017864.
- McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med. 2012 Mar 8;366(10):932-42. doi: 10.1056/NEJMra1007236. Erratum in: N Engl J Med. 2012 May 31;366(22):2138. PMID: 22397655.
- Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, Richard JC, Brochard L. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013 May;39(5):801-10. doi: 10.1007/s00134-013-2823-1. Epub 2013 Jan 24. PMID: 23344830.
- Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, Brochard LJ, Bolz SS, Rubenfeld GD, Kavanagh BP, Ferguson ND. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med. 2015 Apr;41(4):642-9. doi: 10.1007/s00134-015-3687-3. Epub 2015 Feb 19. Erratum in: Intensive Care Med. 2015 Apr;41(4):734. Sebastien-Bolz, Steffen [corrected to Bolz, Steffen-Sebastien]. PMID: 25693448.
- Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A. Coexistence and Impact of Limb Muscle and Diaphragm Weakness at Time of Liberation from Mechanical Ventilation in Medical Intensive Care Unit Patients. Am J Respir Crit Care Med. 2017 Jan 1;195(1):57-66. doi: 10.1164/rccm.201602-0367OC. PMID: 27310484.
- Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and Lung Ultrasound to Predict Weaning Outcome: Systematic Review and Meta-Analysis. Chest. 2017 Dec;152(6):1140-1150. doi: 10.1016/j.chest.2017.08.028. Epub 2017 Aug 31. PMID: 28864053.
- Cavayas YA, Eljaiek R, Rodrigue É, Lamarche Y, Girard M, Wang HT, Levesque S, Denault AY. Preoperative Diaphragm Function Is Associated With Postoperative Pulmonary Complications After Cardiac Surgery. Crit Care Med. 2019 Dec;47(12):e966-e974. doi: 10.1097/CCM.0000000000004027. PMID: 31609771.
- Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011 Mar;55(2):111-5. doi: 10.4103/0019-5049.79879. PMID: 21712864; PMCID: PMC3106380.
- American College of Sports Medicine. Guidelines for Graded Exercise Testing and Exercise Prescription. 3rd ed. Philadelphia: Lea & Febiger,1985.
- Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008 Mar;133(3):737-43. doi: 10.1378/chest.07-2200. Epub 2008 Jan 15. PMID: 18198248.
- Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013 Mar;47(3):319-29. doi: 10.1002/mus.23671. Epub 2013 Feb 4. PMID: 23382111; PMCID: PMC3581727.
- Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, Richard JC, Brochard L. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013 May;39(5):801-10. doi: 10.1007/s00134-013-2823-1. Epub 2013 Jan 24. PMID: 23344830.
- Tuinman PR, Jonkman AH, Dres M, Shi ZH, Goligher EC, Goffi A, de Korte C, Demoule A, Heunks L. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med. 2020 Apr;46(4):594-605. doi: 10.1007/s00134-019-05892-8. Epub 2020 Jan 14. PMID: 31938825; PMCID: PMC7103016.
- National Healthcare Safety Network Team. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event. In: National Healthcare Safety Network Team, editor. National Healthcare Safety Network NHSN Patient Safety Component Manual. Center for Disease Control and Prevention; 2018; 6 –1 to 6 –15.
- Society of Thoracic Surgeons. STS National Database. [cited 2018 Apr 1]. https://www.sts.org/quality-safety/performancemeasures/descriptions#ProlongedIntubation.
- Prasad MK, Sahay S, Varshney RK, Jheetay GS. Evaluation of risk factors for postoperative pulmonary complications after elective open upper abdominal surgery in chronic obstructive pulmonary diseases patients. J Med Soc. 2019; 33: 47-51.
- Avendano CE, Flume PA, Silvestri GA, King LB, Reed CE. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002 Mar;73(3):922-6. doi: 10.1016/s0003-4975(01)03584-6. PMID: 11899202.
- Ferguson MK, Durkin AE. Preoperative prediction of the risk of pulmonary complications after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2002 Apr;123(4):661-9. doi: 10.1067/mtc.2002.120350. PMID: 11986593.
- Karl RC, Schreiber R, Boulware D, Baker S, Coppola D. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg. 2000 May;231(5):635-43. doi: 10.1097/00000658-200005000-00003. PMID: 10767784; PMCID: PMC1421050.
- Doty JR, Salazar JD, Forastiere AA, Heath EI, Kleinberg L, Heitmiller RF. Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann Thorac Surg. 2002 Jul;74(1):227-31; discussion 231. doi: 10.1016/s0003-4975(02)03655-x. PMID: 12118764.
- Sabel MS, Smith JL, Nava HR, Mollen K, Douglass HO, Gibbs JF. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol. 2002 Mar;9(2):210-4. doi: 10.1007/BF02557376. PMID: 11888881.
- Kinugasa S, Tachibana M, Yoshimura H, Dhar DK, Shibakita M, Ohno S, Kubota H, Masunaga R, Nagasue N. Esophageal resection in elderly esophageal carcinoma patients: improvement in postoperative complications. Ann Thorac Surg. 2001 Feb;71(2):414-8. doi: 10.1016/s0003-4975(00)02333-x. PMID: 11235680.
- Arora NS, Rochester DF. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):64-70. doi: 10.1152/jappl.1982.52.1.64. PMID: 7061279.
- Fan ST, Lau WY, Yip WC, Poon GP, Yeung C, Lam WK, Wong KK. Prediction of postoperative pulmonary complications in oesophagogastric cancer surgery. Br J Surg. 1987 May;74(5):408-10. doi: 10.1002/bjs.1800740530. PMID: 3594139.
- Takagi K, Yamamori H, Morishima Y, Toyoda Y, Nakajima N, Tashiro T. Preoperative immunosuppression: its relationship with high morbidity and mortality in patients receiving thoracic esophagectomy. Nutrition. 2001 Jan;17(1):13-7. doi: 10.1016/s0899-9007(00)00504-9. PMID: 11165881.
- Kuwano H, Sumiyoshi K, Sonoda K, Kitamura K, Tsutsui S, Toh Y, Kitamura M, Sugimachi K. Relationship between preoperative assessment of organ function and postoperative morbidity in patients with oesophageal cancer. Eur J Surg. 1998 Aug;164(8):581-6. doi: 10.1080/110241598750005679. PMID: 9720934.
- Johnson RG, Arozullah AM, Neumayer L, Henderson WG, Hosokawa P, Khuri SF. Multivariable predictors of postoperative respiratory failure after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007 Jun;204(6):1188-98. doi: 10.1016/j.jamcollsurg.2007.02.070. PMID: 17544077.
- Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000 Aug;232(2):242-53. doi: 10.1097/00000658-200008000-00015. PMID: 10903604; PMCID: PMC1421137.
- DRIPPS RD, DEMING MV. Postoperative atelectasis and pneumonia. Ann Surg. 1946 Jul;124:94-110. PMID: 20992169.
- Garibaldi RA, Britt MR, Coleman ML, Reading JC, Pace NL. Risk factors for postoperative pneumonia. Am J Med. 1981 Mar;70(3):677-80. doi: 10.1016/0002-9343(81)90595-7. PMID: 7211900.
- Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995 Jun;82(6):1474-506. doi: 10.1097/00000542-199506000-00019. PMID: 7793661.
- Michelet P, D'Journo XB, Roch A, Papazian L, Ragni J, Thomas P, Auffray JP. Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest. 2005 Nov;128(5):3461-6. doi: 10.1378/chest.128.5.3461. PMID: 16304300.
- Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve. 2013 Jun;47(6):884-9. doi: 10.1002/mus.23702. Epub 2013 Apr 29. PMID: 23625789.
- Abou-Jawde RM, Mekhail T, Adelstein DJ, Rybicki LA, Mazzone PJ, Caroll MA, Rice TW. Impact of induction concurrent chemoradiotherapy on pulmonary function and postoperative acute respiratory complications in esophageal cancer. Chest. 2005 Jul;128(1):250-5. doi: 10.1378/chest.128.1.250. PMID: 16002943.
- Lee HK, Vaporciyan AA, Cox JD, Tucker SL, Putnam JB Jr, Ajani JA, Liao Z, Swisher SG, Roth JA, Smythe WR, Walsh GL, Mohan R, Liu HH, Mooring D, Komaki R. Postoperative pulmonary complications after preoperative chemoradiation for esophageal carcinoma: correlation with pulmonary dose-volume histogram parameters. Int J Radiat Oncol Biol Phys. 2003 Dec 1;57(5):1317-22. doi: 10.1016/s0360-3016(03)01373-7. PMID: 14630268.
- Lin FC, Durkin AE, Ferguson MK. Induction therapy does not increase surgical morbidity after esophagectomy for cancer. Ann Thorac Surg. 2004 Nov;78(5):1783-9. doi: 10.1016/j.athoracsur.2004.04.081. PMID: 15511475.
- Papazian L, Fraisse A, Garbe L, Zandotti C, Thomas P, Saux P, Pierrin G, Gouin F. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996 Feb;84(2):280-7. doi: 10.1097/00000542-199602000-00005. PMID: 8602657.
- Arora NS, Rochester DF. Effect of body weight and muscularity on human diaphragm muscle mass, thickness, and area. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):64-70. doi: 10.1152/jappl.1982.52.1.64. PMID: 7061279.
- Arora NS, Rochester DF. Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis. 1982 Jul;126(1):5-8. doi: 10.1164/arrd.1982.126.1.5. PMID: 7091909.
- Nunn JF. Effects of anaesthesia on respiration. Br J Anaesth. 1990 Jul;65(1):54-62. doi: 10.1093/bja/65.1.54. PMID: 2200485.
- Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med. 1994 Jun;149(6):1539-44. doi: 10.1164/ajrccm.149.6.8004310. PMID: 8004310.
- Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol (1985). 2002 Jun;92(6):2585-95. doi: 10.1152/japplphysiol.01213.2001. PMID: 12015377.
- Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol (1985). 2002 May;92(5):1851-8. doi: 10.1152/japplphysiol.00881.2001. PMID: 11960933.
- Anzueto A, Peters JI, Tobin MJ, de los Santos R, Seidenfeld JJ, Moore G, Cox WJ, Coalson JJ. Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med. 1997 Jul;25(7):1187-90. doi: 10.1097/00003246-199707000-00021. PMID: 9233746.
- dos Santos CC, Slutsky AS. Protective ventilation of patients with acute respiratory distress syndrome. Crit Care. 2004 Jun;8(3):145-7. doi: 10.1186/cc2849. Epub 2004 Apr 23. PMID: 15153229; PMCID: PMC468895.
- Tandon S, Batchelor A, Bullock R, Gascoigne A, Griffin M, Hayes N, Hing J, Shaw I, Warnell I, Baudouin SV. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth. 2001 May;86(5):633-8. doi: 10.1093/bja/86.5.633. PMID: 11575337.
- Yano M, Taniguchi M, Tsujinaka T, Fujiwara Y, Yasuda T, Shiozaki H, Monden M. Is preoperative methylprednisolone beneficial for patients undergoing esophagectomy? Hepatogastroenterology. 2005 Mar-Apr;52(62):481-5. PMID: 15816462.
- Sato N, Koeda K, Ikeda K, Kimura Y, Aoki K, Iwaya T, Akiyama Y, Ishida K, Saito K, Endo S. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002 Aug;236(2):184-90. doi: 10.1097/00000658-200208000-00006. PMID: 12170023; PMCID: PMC1422564.
- Nunn JF. Effects of anaesthesia on respiration. Br J Anaesth. 1990 Jul;65(1):54-62. doi: 10.1093/bja/65.1.54. PMID: 2200485.
- Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000 May;92(5):1467-72. doi: 10.1097/00000542-200005000-00037. PMID: 10781293.
- Sharma RR, Axelsson H, Oberg A, Jansson E, Clergue F, Johansson G, Reiz S. Diaphragmatic activity after laparoscopic cholecystectomy. Anesthesiology. 1999 Aug;91(2):406-13. doi: 10.1097/00000542-199908000-00014. PMID: 10443603.
- Ford GT, Rosenal TW, Clergue F, Whitelaw WA. Respiratory physiology in upper abdominal surgery. Clin Chest Med. 1993 Jun;14(2):237-52. PMID: 8519170.