More Information
Submitted: May 21, 2025 | Approved: May 26, 2025 | Published: May 27, 2025
How to cite this article: Khulbe KC, Matsuura T, Savoji H. Recent Development of Nanofibers in Medical/Pharmaceutical Sectors. Arch Surg Clin Res. 2025; 9(1): 008-016. Available from:
https://dx.doi.org/10.29328/journal.ascr.1001085.
DOI: 10.29328/journal.ascr.1001085
Copyright license: © 2025 Khulbe KC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nanofibers; Drug delivery; Organ-on-a-chip technology; Wound dressing
Recent Development of Nanofibers in Medical/Pharmaceutical Sectors
KC Khulbe1*, T Matsuura1 and H Savoji2
1Industrial Membrane Research Laboratory, Department of Chemical and Biological Engineering, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada
2Institute of Biomedical Engineering, Department of Pharmacology and Physiology, Faculty of Medicine, University of Montreal, Montreal, Canada
*Address for Correspondence: KC Khulbe, Industrial Membrane Research Laboratory, Department of Chemical and Biological Engineering, University of Ottawa, Ottawa, Ontario K1N 6N5, Canada, Email: [email protected]
This article provides a comprehensive overview of the properties, applications, and fabrication techniques of nanofibers, which are characterized by their ultrafine diameters and unique features such as high surface area and aspect ratio. These attributes render nanofibers particularly advantageous for a wide range of applications, especially in the biomedical sector, encompassing areas like tissue engineering, drug delivery, and wound dressing. The article highlights various studies that illustrate the potential of nanofibers in addressing healthcare challenges, particularly their utilization in scaffolds for regenerative medicine and as carriers for controlled drug delivery. Furthermore, it discusses different preparation methods for nanofibers, including electrospinning and alternative techniques, while stressing the importance of polymer selection in achieving optimal drug-release properties. The article also delves into the application of nanofibers in tissue engineering, specifically for bone, cartilage, and vascular applications, and examines their emerging roles in organ-on-a-chip technology and contraceptive development. In conclusion, the article emphasizes the versatility and significance of nanofibers in advancing medical technologies and their potential to address contemporary health challenges. Collaborative efforts between material scientists and biologists are essential to foster interdisciplinary research aimed at improving electrospinning methodologies.
Nanofibers have found extensive applications across various industries and fields, including biomedical and healthcare, environmental remediation, textiles and apparel, electronics, and energy. In the biomedical sector, nanofibers play a pivotal role in tissue engineering, drug delivery systems, wound dressings, and scaffolds for regenerative medicine. Their unique properties and adaptable nature render nanofibers invaluable in advancing technological innovations and addressing contemporary challenges across multiple domains.
Numerous studies have substantiated the immense potential of nanofibers in tackling current challenges within the medical and healthcare sectors. Rasouli, et al. [1] discussed nanofiber technologies, unique properties, fabrication techniques (including physical, chemical, and biological methods), and emerging applications in biomedical and healthcare fields. Ghajarieh, et al. [2] summarized the applications of nanofibers in various biomedical areas, such as tissue engineering, wound dressing, and facemasks, highlighting opportunities to develop new materials and techniques that enhance the ability to create rapid, sensitive, and reliable analytical methods. Sharma, et al. [3] examined the challenges and opportunities facing multifunctional nanofibers for active therapeutic applications. Toriello, et al. [4] presented recent advancements in the fabrication and applications of electrospun nanofiber composites, demonstrating the outstanding potential of composite nanofibers for biomedical applications. Cui, et al. [5] provided a comprehensive summary of electrospinning techniques that regulate the composition and structure of nanofibrous materials. These materials have potential applications in various fields, including skin, blood vessels, the nervous system, and bone tissue engineering, as well as drug delivery.
Polymeric membranes
The primary advantage of polymeric compounds lies in their ability to offer a wide range of chemical and physical characteristics. Optimal polymeric membranes, influenced by factors such as the structure of monomers, crystallinity percentage, molecular weight of the polymer, and type, can be achieved through blending with other polymers. Numerous membrane polymers are either grafted, custom-modified, or synthesized as copolymers to enhance their properties. The most prevalent polymers utilized in membrane synthesis include cellulose acetate, nitrocellulose, and cellulose esters (CA, CN, and CE), as well as polysulfone (PSf), polyether sulfone (PES), polyacrylonitrile (PAN), polyamide, polyimide, polyethylene, polypropylene (PE and PP), polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), and saccharides.
Biodegradable polymers
Polymers can be categorized into two main types: biodegradable and non-biodegradable, based on their biodegradability. Biodegradable polymers play a crucial role in various pharmaceutical and biomedical applications due to their low toxicity and compatibility with biological tissues and cells. Among these, cellulose is recognized as the first natural biopolymer known to humanity [6]. Bacterial cellulose (BC) represents a highly pure form of cellulose, produced as a swollen membrane by certain bacteria [7]. Research by Lazarini, et al. [8] highlighted the significance of BC membranes as a support for antimicrobial sustained release in skin wound treatment. The biocompatibility of BC makes it an exceptionally desirable material for wound healing applications [9]. Additionally, pullulan, a naturally occurring substance derived from the fungus Aureobasidium pullulans, is sometimes called 'edible packaging.' This polysaccharide polymer consists of maltotriose units, also known as α-1,4-; α-1,6-glucan. Pullulan possesses unique characteristics, being water-soluble, non-mutagenic, non-immunogenic, and non-toxic, making it an important biopolymer.
Due to the unique properties of gelatin and the distinctive characteristics of electrospun nanofibers, electrospun gelatin nanofibers (GNFs) represent optimal materials for transdermal or topical drug delivery, wound dressings, and as carriers and scaffolds for cell and tissue culture. A significant application of gelatin is in biomineralization, which refers to the synthesis of nanomaterials aimed at various biomedical applications, including drug and cell therapy engineering, cancer/tumor targeting, and bone tissue engineering [10] Natural polysaccharides, including alginate, pullulan, hyaluronic acid, dextran, cellulose, chondroitin sulfate, chitosan, xanthan gum, and gellan gum, are examined by Priya, et al. [11] for their properties, pharmaceutical applications, and biomedical uses.
Preparation of nanofibers
Several techniques for creating nanofibers include electrospinning, which is preferred for its high surface area to volume ratio [12]. Key methods of electrospinning are:
- Coaxial electrospinning: Produces core-sheath nanofibers.
- Multi-jet electrospinning: Designed for large-scale production.
- Emulsion electrospinning: A quick and cost-effective method for core-shell nanofibers.
- Bubble electrospinning: Uses electrical forces to manipulate bubbles.
- Roller electrospinning: A needleless method using rollers for fiber preparation.
- Electrospinning by a porous hollow tube: Enhances efficiency with a porous wall tube.
Other methods for producing nanofibers include milling, Physical Vapor Deposition (PVD), laser ablation, and spin fabrication. Electrospinning technology is a technique that uses electrostatic forces to spray polymer solutions to form nanofibers. The advantages of nanofibers produced via electrospinning exhibit distinctive characteristics, encompassing a substantial surface area, a diverse range of matrix materials, the capacity for loading and delivering various drugs, a microstructure akin to the ECM, and high porosity with interconnectivity.
Wound dressing
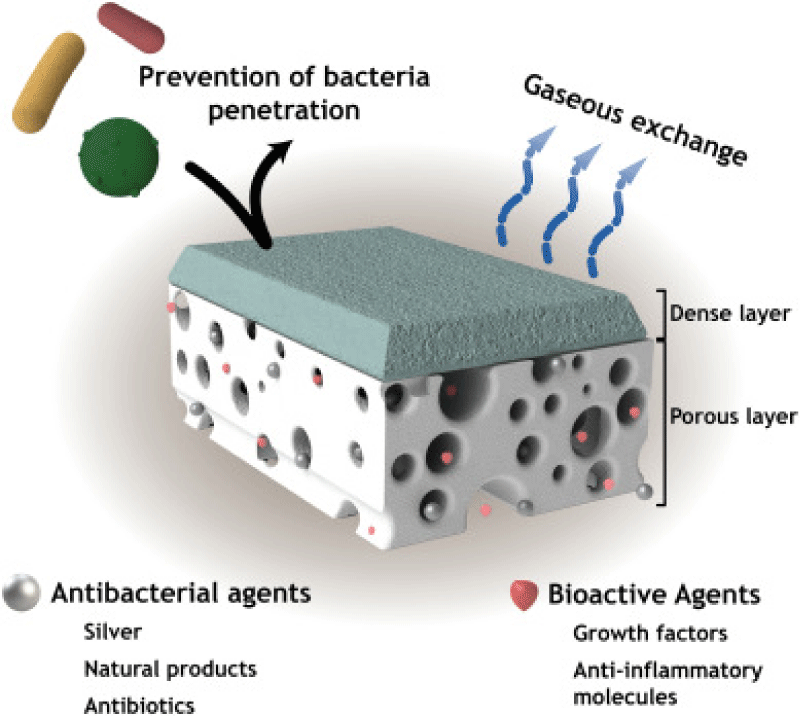
Researchers have developed nanofiber-based wound dressings that significantly enhance the wound healing process by creating an optimal environment for tissue regeneration. A recent study published in the journal "Acta Biomaterialia" provides a comprehensive review of nanofiber dressings infused with essential oils for treating various types of wounds [13]. This study emphasizes the application of electrospun nanofibers in wound healing. The primary characteristics and functionalities of these asymmetric membranes used in wound dressing applications are illustrated in Figure 1.
Figure 1: Illustration of the main features displayed by asymmetric membranes aimed to improve wound healing [14].
Recent studies on wound healing applications of nanofiber membrane show that electrospun composite membrane is promising in anti-infective therapy [15], chronic wound treatment [16], and skin reparation [17]. Alyamani, et al. [18] reported on the use of polycaprolactone/chitosan (PCL/CH) nanofibers infused with Cordia myxa fruit extract (CMFE) as a potential biocompatible antibacterial agent for wound dressings. Their study highlights the effectiveness of these nanofibers in providing antimicrobial properties, making them a promising option for enhancing wound care. Doxycycline (DCH) is a broad-spectrum antibiotic and matrix metalloproteinases inhibitor, which has prominent efficacy for chronic wound treatment. DCH/PLA nanofiber mats hold great potential as wound dressings for chronic wound treatment [5] (Table 1).
| Table 1: Shows the therapeutic agents incorporated into nanofibers to improve wound healing [19]. | ||
| Therapeutic Agents | Nanofibers | Purpose |
| Lysozyme | Chitosan/PVA | Antibacterial |
| Silver | Gelatin/polyurethane; gelatin; polyurethane; poly(ethylene-co-vinyl alcohol) | Antibacterial |
| ZnO | PCL; alginate/PVA | Antibacterial |
| Cefoxitin sodium | PLGA | Antibacterial |
| Gentamicin | Chitosan | Antibacterial |
| Ciprofloxacin HCl | Polyurethane/dextran; PVA/poly(vinyl acetate) | Antibacterial |
| Polyhexamethylene biguanide | Cellulose acetate/polyester urethane | Antibacterial |
| Lidocaine, mupirocin | PLLA | Pain management and antibacterial |
| Fibrinogen | PLLA | Hemostasis |
| Curcumin | PCL | Antioxidant |
| VEGF | Chitosan/PEO; HA/collagen | Angiogenesis |
| PDGF-BB | Polyurethane; HA/collagen | Angiogenesis, granulation tissue formation |
| EGF | PCL–PEG/PCL; poly(l-lactic acid)-co-poly-(ε-caprolactone); HA/collagen; PCL/PEG | Keratinocyte migration and maturation, angiogenesis |
| Basic-FGF | PELA; HA/collagen | Cell adhesion, proliferation, ECM secretion, re-epithelialization, and skin appendages regeneration, angiogenesis |
| Abbreviations: ECM: Extracellular Matrix; HA: Hyaluronic Acid; PCL: Polycaprolactone; PELA: Poly(Ethylene Glycol-Co-Lactide); PEO: Polyethylene Oxide; PLLA: Poly-L-Lactic-Acid; PVA: Polyvinyl Alcohol | ||
Drug delivery
Nanofibers are being explored as carriers for controlled drug delivery. They can release drugs at a controlled rate, improving patient compliance and reducing side effects. Zhu, et al. [20] discussed the use of nanofiber-based drug delivery systems. Nanofibers for modified drug release are categorized based on characteristics such as prolonged, stimulus-activated, and biphasic release. In general, swellable or degradable polymers are used to modify the drug release. The formulation of nanofibers is very complex and depends on many variables, although at the same time, the design of the formulation offers many opportunities to achieve the desired nanofiber drug-release properties [21].
To achieve the desired drug-release properties, it is essential to select the appropriate polymer(s). For immediate drug release, a water-soluble polymer is recommended. For prolonged drug release, a swellable or degradable polymer should be considered. Additionally, a stimulus-responsive polymer can be utilized for stimulus-activated drug release. The choice of solvent and the electrospinning setup, such as single nozzle or coaxial electrospinning, are also critical factors in this process.
Tissue engineering
Tissue engineering encompasses a diverse array of applications, primarily associated with the repair or replacement of specific tissues or entire organs, including but not limited to bone, cartilage, blood vessels, bladder, skin, and muscle. The methodologies employed in tissue engineering often involve the utilization of scaffolds in conjunction with biological supplements and cellular components. Notable references in this field include works by Ye, et al. [22], which highlight significant advancements in the application of these technologies. Furthermore, nanofiber-based scaffolds have emerged as pivotal tools in various tissue engineering applications, facilitating the regeneration of bones, cartilage, ligaments, skeletal muscles, skin, blood vessels, and neural tissues, as discussed by Kumar, et al. [23] in their comprehensive analysis of nanomaterials in this domain. Gabriel, et al. [24] fabricated electrospun polyurethane membranes for tissue engineering such as epithelial, drug delivery, or cardiac applications.
Vascular tissue engineering: Cardiovascular diseases (CVDs) are the leading cause of death globally, taking an estimated 17.9 million lives each year. CVDs are a group of disorders of the heart and blood vessels and include coronary heart disease, cerebrovascular disease, rheumatic heart disease, and other conditions [25]. To effectively replicate the biological and mechanical properties of autologous vessels, a small-diameter, tissue-engineered vascular graft (TEVG) is required [23,26]. Electrospinning is a promising technique for developing tissue-engineered vessel grafts (TEVGs). Electrospun nanofibers are excellent fabrication materials for arterial TEVG applications, as they provide desired mechanical properties, elasticity, biocompatibility, bioactivity, and antithrombotic scaffold characteristics [27].
Cuenca, et al. [28] developed vascular grafts using electrospun polycaprolactone (PCL) combined with a decellularized rat aorta matrix (ECM), followed by the incorporation of heparin and vascular endothelial growth factor (VEGF). In vitro studies demonstrated that the PCL/ECM/VEGF vascular grafts exhibited excellent hemocompatibility and biocompatibility. The authors suggested that these grafts should be considered for potential applications in small-diameter vascular grafts within clinical settings. Di Francesco, et al. [29] discussed the progress in making tissue-engineered blood vessels and using natural materials to support the creation of these vessels.
Bone tissue engineering: Tissue damage related to bone and cartilage is a common clinical disease. The primary connective tissues of the human body that carry weight are bones. They are highly susceptible to deterioration. Bone abnormality is the most challenging and has been an important research topic. Recently, the study of scaffolds for bone, cartilage, and osteochondral tissue engineering has become more advanced and is getting more attention [26]. Raja, et al. [30], elaborated on the importance of polyphenol-loaded electrospun nanofibers in bone tissue regeneration and discussed the possible challenges and future directions in this field.
Skin tissue engineering: Skin is the body's outermost layer and the largest organ of the integumentary system. There has been a lot of recent research on biomimetic nanoparticles that can help to regenerate injured tissues. Skin tissue engineering (STE) is an effective strategy for skin regeneration, utilizing synthetic biomaterials with favorable fibrillar characteristics. However, these materials often lack in situ degradation. Recent advancements include hydrogels, nanofiber scaffolds, and 3D-printed composites, with electrospun nanofiber scaffolds gaining attention for their availability, resemblance to natural structures, and regenerative capabilities. Despite their potential, improvements in degradation and mechanical properties are needed for clinical effectiveness [31-33].
Electrospun fibers with a high surface area to volume ratio and structures mimicking extracellular matrix (ECM) have shown great potential in tissue engineering applications. The ability of electrospun nanofibers to mimic the structure and composition of certain components of the extracellular matrix (ECM) has been widely used in the construction of tissue regeneration scaffolds [34].
Cartilage tissue engineering: The primary cause of disability is cartilage disintegration, which can be brought on by trauma or common joint conditions like osteoarthritis. Nanofibers are vital in managing cartilage tissue regeneration [26]
Cartilage is a vital tissue in both animals and humans, but it lacks nerves, blood vessels, and lymphatics, making its healing and repair complex. In tissue engineering, 'repair' involves replacement and regeneration. Replacement restores severely damaged tissues through connective tissue deposition. Nanomaterials offer opportunities for scaffolds that mimic the extracellular matrix (ECM) of cartilage, enhancing cell interaction and tissue functionality. Innovative nanotechnology methods like 3-D fiber deposition and electrospinning improve nanoscaffold quality. Nanofibers from electrospinning are widely used in cartilage engineering, while polymer-hydroxyapatite nanocomposites show potential for cartilage regeneration. Natural materials like polysaccharide-based and protein-based scaffolds are crucial for effective cartilage repair [35].
Nanomaterials show promise in creating scaffolds that better mimic the extracellular matrix (ECM) of cartilage, enhancing cell interaction and engineered-tissue functionality [36,37].
Hemodialysis (artificial kidney)
The kidney is a key component of the body's waste disposal and acid-base regulation mechanisms. In hemodialysis, membranes are used as artificial kidneys to remove toxins from patients' blood, such as urea, creatinine, uric acid, and other compounds that are usually eliminated by the kidneys through the formation of urine. The use of various nanomaterials (including carbon nanotubes, nanofibrous membranes), mesenchymal stem cells-derived nanovesicles, and nanomaterial-based adsorbents and membranes that are used in wearable blood purification systems and synthetic kidneys [32].
Blood oxygenation (artificial lung)
Polymeric membranes have extensive applications as oxygenators or for hemodialysis. Membrane oxygenators with advanced characteristics usually use aromatic polyimide, silicone-coated polypropylene (PP), poly-4-methylpentene-1 (PMP), and polydimethylsiloxane (PDMS) copolymer hollow fiber membranes. Oxygenators are a vital component of respiratory support devices, initially developed as a heart-lung machine for treating cardiac diseases. Hollow fiber membranes are widely used in oxygenators due to their remarkable efficiency in facilitating the exchange of oxygen and carbon dioxide with the bloodstream. Extracorporeal membrane oxygenation (ECMO) has become the standard treatment for advanced acute heart and respiratory failure. A key feature of an Extracorporeal Life Support (ECLS) circuit is the integration of a membrane oxygenator, which is synonymous with ECMO. Furthermore, a miniaturized oxygenator, also known as a microfluidic membrane oxygenator, is created using advanced microfabrication techniques for artificial lung applications. These devices consist of three essential components: the blood side flow manifold, the gas side flow manifold, and a thin gas-permeable membrane. Membrane oxygenators with advanced characteristics typically utilize materials such as aromatic polyimide, silicone-coated polypropylene (PP), poly-4-methylpentene-1 (PMP), and polydimethylsiloxane (PDMS) copolymer hollow fiber membranes [38].
Artificial pancreas
Pancreatic regeneration is increasingly recognized as a potential treatment for diabetes mellitus, pancreatic cancer, and pancreatic dysfunction. The development of a bioartificial pancreas began in 1933 when tissue containing insulin-secreting cells was first transplanted as a potential diabetes treatment [39]. Nanostructured materials serve as three-dimensional scaffolds that can replicate the physiological characteristics of the natural extracellular matrix of the pancreas. This replication facilitates the anabolic activities of pancreatic islets through the regulation, differentiation, and proliferation of beta cells within the pancreatic microenvironment.
Scaffolds at the nanometer scale, such as nanoparticles, nanofibers, and nanocomposites, are promising in biomedical and clinical applications. Oran, et al. discuss the current state of nanostructured biomaterials and their applications in pancreatic regeneration [40]. Two common designs of bio-artificial pancreas using membranes are hollow fiber and micro-encapsulation [41]
Cancer therapy
Cancer cells are surrounded by a dynamic fluid-mosaic membrane that functions as both a barrier and a communication filter. Cell membrane-coated nanoparticles (CMNPs) have demonstrated significant potential in the precise targeting and delivery of therapeutic agents to cancerous sites. Electrospun nanofiber-derived scaffolds offer a promising platform for cancer therapy, facilitating the targeted delivery of therapeutic agents while minimizing adverse effects on healthy tissues. Ongoing research is focused on the application of electrospun nanofibers across various cancer treatment modalities, including chemotherapy and gene therapy. Xu, et al. [42], developed a device utilizing hyaluronic acid (HA) and polyethyleneimine (PEI)-modified electrospun poly (lactic-co-glycolic acid) (PLGA) nanofibers for the capture and culture of cancer cells, as detailed in their study published in Biomaterials Science.
Electrospun nanofiber-derived scaffolds emerge as a promising platform for cancer therapy, allowing the delivery of therapeutic components in the target sites, minimizing adverse effects on normal tissues, and offering functional versatility. Kuang, et al. [43] provides guidance on nanofibers-derived scaffolds for cancer therapy, and explores state-of-the-art research on electrospun nanofibers or their derived composite scaffolds in the realm of cancer treatment, and various modalities are employed, covering chemotherapy, photodynamic therapy, thermal therapy, gene therapy, antitumor combination therapy, and multifunctional therapy.
Organ-on-a-chip technology
Organ-on-a-chip membranes have gained traction as a promising technology for research in tissue engineering, drug discovery, and precision medicine. Current investigations focus on constructing various organ-on-a-chip systems, which simulate the functions of human organs, including lung, kidney, gut, and brain models. The integration of nanofiber technology in these systems is expected to enhance their functionality and mimic real tissue characteristics.
As highlighted in the study by Corral-Nájera, et al. [44], these polymeric and biological membranes are pivotal for the development of organ-on-a-chip devices. The anticipated applications of organ-on-a-chip technology in preclinical research and therapeutic testing are significant. Current investigations are focused on the fabrication of various organ-on-a-chip models, including lung-on-a-chip, kidney-on-a-chip, gut-on-a-chip, and brain-on-a-chip systems. The integration of fiber materials in the construction of organ-on-a-chip devices, whether utilized as a three-dimensional scaffold for cell culture or to more accurately replicate the characteristics of real tissues and organs, presents innovative approaches for the advancement of novel organ-on-a-chip systems, as discussed by Yang, et al. [45], in their exploration of micro- and nanofibers for organ-on-a-chip applications. Furthermore, nanofiber membranes play a crucial role in the development of organ-on-a-chip devices that replicate the functionalities of human organs, encompassing applications such as lung-on-a-chip, heart-on-a-chip, liver-on-a-chip, nerve-on-a-chip, and multi-organ-on-a-chip systems.
Xu, et al. [42] developed a sophisticated device utilizing hyaluronic acid (HA) and polyethyleneimine (PEI) modified electrospun poly (lactic-co-glycolic acid) (PLGA) nanofibers for the capture and culture of cancer cells. Yang, et al. [46], developed a lung-on-a-chip with a poly (lactic-co-glycolic acid) (PLGA) electrospinning nanofiber membrane as the chip substrate and cell scaffold for lung cancer. Table 2 shows a few applications of microfibers in organ-on-a-chip construction
| Table 2: Application of microfibers in organ-on-a-chip construction. | ||||||
| Type of Chip | Materials | Fiber Manufacturing Method & Size | Cell Type | Cell Loading Method | Point | Ref |
| Nerve-on-a-chip | Alginate, GelMA | Capillary-based; Diameter: 200/400 μm | Schwann cell | Inner wall of hollow microfibers | Study the relationship between nerve cell proliferation in microfibers | [47] |
| Islet-on-a-chip | Alginate, GelMA | Microchip-based; Cross-section: 390–720 μm, Cavity: 140–360 μm | MS1, β-TC6 | Inner wall of hollow microfibers | Achieve vascularization and evaluate sugar-substituted insulin and glucagon secretion | [48] |
| Nerve-on-a-chip | Alginate, GelMA | Microchip-based | HUVEC, PC12 | Inner wall of hollow microfibers | Construct a neural model based on HUVEC-loaded hollow microfibers | [49] |
Contraceptives
Recent studies have highlighted the potential of vaginal nanofibers in addressing diseases such as cancer and infections. Researchers are actively exploring the formulation of nanocarriers for vaginal drug delivery. Different nanoplatforms based on drugs, peptides, proteins, antigens, hormones, nucleic material, and microbicides are gaining momentum for vaginal therapeutics [50].
Misleneous
Kharaziha, et al. [51] developed novel nanofibrous membranes through electrospinning of PCL-forsterite nanopowder. It was reported that composite nanofibrous membranes possessed significantly improved cellular responses in terms of attachment, proliferation and mineralization of pre-osteoblasts compared to PCL membrane. Thus, the currently developed nanofibrous composite membranes embedded in forsterite nanopowder expected to be attractive in GBR (guided bone regeneration) membrane applications. Xu, et al. suggested that three-dimensional (3D) monolithic structures (i.e., aerogels/sponges/scaffolds) from fragmented electrospun nanofiber mats/membranes can be obtained, and it can be used in biomedical engineering (e.g., tissue engineering, hydrogel, and drug delivery). Table 3 shows few recent use of nanofiber/mats/membranes applications in medical field.
| Table 3: Few recent use of nanofiber/mats/membranes applications in medical field. | ||
| Membrane | Application | Ref. |
| Polyacrylonitrile/MoS2 composite nanofibers | Growth behavior of bone marrow mesenchymal stem cells | Wu, et al. 2018 [53] |
| Biomimetic Domain-Active Electrospun Scaffolds | Facilitating Bone Regeneration Synergistically with Antibacterial Efficacy for Bone Defects. | Qian, et al. 2018 [54] |
| N-maleoyl-functional chitosan (MCS)/PEO NFMs (nanofiber meshes) | Utilized as tissue scaffolds, especially for functional wound healing dressings. | Chen, et al. 2020 [55] |
| PEO-chitosan nanofibers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline | Cancer treatment | Rengifo, et al. 2019 [56] |
| Tri-layered composite nanofiber scaffold (PVA-PVAc loaded with simvastatin / PCL-CA-β-tcp/PCL | Drug release Bone tissue regeneration |
Rezk, et al. 2018 [57] |
| Nanofibrous silk fibroin/reduced graphene oxide scaffolds | Tissue engineering and cell culture | Nalvuran, et al. 2018 [58] |
| Polyurethane/graphene nanocomposite | Biomedical (tissue engineering) | Bahrami, et al. [59] |
| Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles | Wound dressing | Garcia-Orue, et al. 2019 [60] |
| Chitosan based nanofiber (Chitosan/ silver nanoparticles and another one with cinnamaldeyhde) | Wound healing and tissue regeneration applications. | Cremar, et al. 2018 [61] |
| Electrospun polyurethane nanofibrous composite impregnated with metallic copper | Wound healing | Jaganathan, et al. 2018 [62] |
| Polyhydroxybutyrate-co- hydroxyvaletare (PHBV) nanofibrous scaffolds containing treated bredigite (T-BR) |
Bone Tissue Engineering | Kouhi, et al. 2019 [63] |
| Composite nanofibers of magnesium oxide (MgO), poly (ε-caprolactone)(PCL) and chitosan (CS) |
Tissue Engineering | Rijal, et al. 2018 [64] |
| Chitosan/polyurethane (CSP) nanofibrous membrane incorporated silver nanoparticles (AgNPs) within the membrane. |
Dental barrier membrane | Lee, et al. 2018 [65] |
| Multifunctional PVA/ZnO nanofibers composite membranes | Surgical gown Application | Khan, et al. 2019 [66] |
| Electrospun poly(lactic acid)/poly(butylene carbonate)/graphene oxide nanofiber membranes | Antibacterial applications | Gu, et al. 2019 [67] |
| Starch-based nanofibrous scaffolds | Wound healing | Waghmare, et al. 2018 [68] |
| Carbon nanotubes- silver nanoparticles- polyvinyl alcohol nanofibers. | Antibacterial wound dressing | Jatoi, et al. 2019 [69] |
| PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane. | Guided bone regeneration | Ye, et al. 2019 [70] |
| Electrospun composite scaffold of poly(3-hydroxybutyrate)-chitosan/alumina nanowires | Bone tissue engineering | Toloue, et al. 2019 [71] |
| Ciprofloxacin-Loaded Povidone Foils and Nanofiber Mats | Delivery and Efficacy of in a Wound-Infection Model Based on Ex Vivo Human Skin. | Rancan, et al. 2019 [72] |
| Gelatin/PCL/ chondroitin sulfate (CS) composite nanofiber | Vascular tissue engineering | Kong, et al. 2021 [73] |
| Fe3O4/ poly-L-lactide) PLLA (nanofibers | Bone tissue engineering | Lai, et al. 2018 [74] |
The article offers an extensive examination of the properties, applications, and fabrication techniques of nanofibers, which are defined by their ultrafine diameters and unique characteristics, such as high surface area and aspect ratio. These features make nanofibers particularly beneficial for a diverse array of applications, particularly in the biomedical field, which includes areas like tissue engineering, drug delivery, and wound dressing. The article showcases numerous studies that demonstrate the potential of nanofibers in addressing challenges within healthcare, particularly their use in scaffolds for regenerative medicine and as carriers for controlled drug delivery. Additionally, it elaborates on various preparation methods for nanofibers, including electrospinning and alternative techniques, while emphasizing the crucial role of polymer selection in achieving optimal drug-release properties. The article further explores the application of nanofibers in tissue engineering, specifically for bone, cartilage, and vascular applications, and investigates their emerging roles in organ-on-a-chip technology and contraceptive development. In conclusion, the article underscores the versatility and importance of nanofibers in the progression of medical technologies and their potential to tackle contemporary health challenges.
Currently, despite the promise of electrospun fibrous materials, in vivo investigations remain notably limited. For drug delivery applications, it is essential to develop more advanced electrospinning techniques that are specifically tailored to the requirements of biomedical applications. Collaborative efforts between material scientists and biologists are vital to promote interdisciplinary research aimed at enhancing electrospinning methodologies.
- Rasouli R, Barhoum A, Bechelany M, Dufresne A. Nanofibers for biomedical and healthcare applications. Macromol Biosci. 2018;19(2):1800256. Available from: https://doi.org/10.1002/mabi.201800256
- Ghajarieh A, Habibi S, Talebian A. Biomedical applications of nanofibers. Russ J Appl Chem. 2021;94(7):847–72. Available from: https://link.springer.com/article/10.1134/S1070427221070016
- Sharma J, Lizu M, Stewart M, Zygula K, Lu Y, Chauhan R, et al. Polymers. 2015;7:186–219. Available from: https://doi.org/10.3390/polym7020186
- Toriello M, Afsari M, Shon HKK, Tijing LD. Progress on the fabrication and application of electrospun nanofiber composites. Membranes (Basel). 2020;10(9):204. Available from: https://doi.org/10.3390/membranes10090204
- Cui W, Zhou Y, Chang J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci Technol Adv Mater. 2010;11(1):014108. Available from: https://doi.org/10.1088/1468-6996/11/1/014108
- Rezaei A, Nasirpour A, Fathi M. Application of cellulosic nanofibers in food science using electrospinning and its potential risk. Compr Rev Food Sci Food Saf. 2015;14(3):269–84. Available from: https://doi.org/10.1111/1541-4337.12128
- Almeida IF, Pereira T, Silva NHCS, Gomes FP, Silvestre AJD, Freire CSR, et al. Bacterial cellulose membranes as drug delivery systems: an in vivo skin compatibility study. Eur J Pharm Biopharm. 2014;86(3):332–6. Available from: https://doi.org/10.1016/j.ejpb.2013.08.008
- Lazarini SC, Yamada C, Barud HS, Trovatti E, Corbi PP, Lustri WR. Influence of chemical and physical conditions in selection of Gluconacetobacter hansenii ATCC 23769 strains with high capacity to produce bacterial cellulose for application as sustained antimicrobial drug-release supports. J Appl Microbiol. 2018;125(3):777–91. Available from: https://doi.org/10.1111/jam.13916
- Ahmed J, Gultekinoglu M, Edirisinghe M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol Adv. 2020;41:107549. Available from: https://doi.org/10.1016/j.biotechadv.2020.107549
- El-Seedi HR, Said NS, Yosri N, Hawash HB, El-Sherif DM, Abouzid M, et al. Gelatin nanofibers: recent insights in synthesis, bio-medical applications and limitations. Heliyon. 2023;9:e16228. Available from: https://doi.org/10.1016/j.heliyon.2023.e16228
- Priya S, Batra U, Samshritha RN, Sharma S, Chaurasiya A, Singhvi G. Polysaccharide-based nanofibers for pharmaceutical and biomedical applications: a review. Macromolecules. 2022;218:209–24. Available from: https://doi.org/10.1016/j.ijbiomac.2022.07.118
- Hiwrale A, Bharati S, Pingale P, Rajput A. Nanofibers: a current era in drug delivery system. Heliyon. 2023;9(9):e18917. Available from: https://doi.org/10.1016/j.heliyon.2023.e18917
- Kharaziha M, Fathi M, Edrisy A, Marashi SM. Nanofiber dressings containing essential oils for the treatment of different types of wounds: a review. Acta Biomater. 2021;126:46–66.
- Miguel SP, Moreira AF, Correia IJ. Chitosan based-asymmetric membranes for wound healing: a review. Int J Biol Macromol. 2019;127:460–75. Available from: https://doi.org/10.1016/j.ijbiomac.2019.01.072
- Zhang S, Ye J, Sun Y, Kang J, Liu J, Wang Y, et al. Electrospun fibrous mat based on silver (I) metal-organic frameworks-polylactic acid for bacterial killing and antibiotic-free wound dressing. Chem Eng J. 2020;390:Article 124523. Available from: https://doi.org/10.1016/j.cej.2020.124523
- Cui S, Sun X, Li K, Gou D, Zhou Y, Hu J, et al. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater Sci Eng C. 2019;104:109745. Available from: https://doi.org/10.1016/j.msec.2019.109745
- Sandri G, Rossi S, Bonferoni MC, Miele D, Faccendini A, Del Favero E, et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr Polym. 2019;220:219–27. Available from: https://doi.org/10.1016/j.carbpol.2019.05.069
- Alyamani AA, Al-Musawi MH, Albukhaty S, Sulaiman GM, Ibrahim KM, Ahmed EM, et al. Electrospun polycaprolactone/chitosan nanofibers containing Cordia myxa fruit extract as potential biocompatible antibacterial wound dressings. Molecules. 2023;28:2501. Available from: https://doi.org/10.3390/molecules28062501
- Chen S, Liu B, Carlson MA, Gambart AF, Reilly DA, Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017;12(11):1335–52. Available from: https://doi.org/10.2217/nnm-2017-0017
- Zhu Z, An Z, Chen L. Advances in electrospun nanofibers for drug delivery. Adv Drug Deliv Rev. 2019;143:1–19.
- Kajdič S, Planinšek O, Gašperlin M, Kocbek P. Electrospun nanofibers for customized drug-delivery systems. J Drug Deliv Sci Technol. 2019;51:672–81. Available from: http://dx.doi.org/10.1016/j.jddst.2019.03.038
- Ye K, Kuang H, You Z, Morsi Y, Mo X. Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics. 2019;11(4):18. Available from: https://doi.org/10.3390/pharmaceutics11040182
- Kumar V, Naqvi S, Gopinath P. Applications of nanofibers in tissue engineering. In: Applications of Nanomaterials: Advances and Key Technologies. Micro and Nano Technologies. 2018;179–203.
- Gabriel LP, Rodrigues AM, Macedo M, Jardini AL, Filho RM. Electrospun polyurethane membranes for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2017;72:113–7. Available from: https://doi.org/10.1016/j.msec.2016.11.057
- Cardiovascular diseases - World Health Organization (WHO). 2023 Sep 19. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Ding H, Cheng Y, Niu X, Hu Y. Application of electrospun nanofibers in bone, cartilage and osteochondral tissue engineering. J Biomater Sci Polym Ed. 2020;32(4):536–61. Available from: https://doi.org/10.1080/09205063.2020.1849922
- Fukunishi T, Shoji T, Shinoka T. Nanofiber composites in vascular tissue engineering. In: Nanofiber Composites for Biomedical Applications. 2017;455–81.
- Cuenca JP, Kang HJ, Fahad MAA, Park M, Choi M, Lee HY, et al. Physico-mechanical and biological evaluation of heparin/VEGF-loaded electrospun polycaprolactone/decellularized rat aorta extracellular matrix for small-diameter vascular grafts. J Biomater Sci Polym Ed. 2022;33:1664–84. Available from: https://doi.org/10.1080/09205063.2022.2069398
- Di Francesco D, Pigliafreddo A, Casarella S, Di Nunno L, Mantovani D, Boccafoschi F. Biological materials for tissue-engineered vascular grafts: overview of recent advancements. Biomolecules. 2023;13(9):1389. Available from: https://doi.org/10.3390/biom13091389
- Raja IS, Preeth DR, Vedhanayagam M, Hyon SH, Lim D, Kim B, et al. Polyphenols-loaded electrospun nanofibers in bone tissue engineering and regeneration. Biomater Res. 2021;25:29. Available from: https://doi.org/10.1186/s40824-021-00229-3
- Meng Q, Li Y, Wang Q, Wang Y, Li K, Chen S, et al. Recent advances of electrospun nanofiber-enhanced hydrogel composite scaffolds in tissue engineering. J Manuf Process. 2024;123:112–27. Available from: http://dx.doi.org/10.1016/j.jmapro.2024.05.085
- Eftekhari A, Maleki Dizaj S, Ahmadian E, Przekora A, Hosseiniyan Khatibi SM, Ardalan M, et al. Application of advanced nanomaterials for kidney failure treatment and regeneration. Materials (Basel). 2021;14(11):2939. Available from: https://doi.org/10.3390/ma14112939
- Wu J, Yu F, Shao M, Zhang T, Lu W, Chen X, et al. Electrospun nanofiber scaffold for skin tissue engineering (review). ACS Appl Bio Mater. 2024;7(6):3556–67. Available from: https://doi.org/10.1021/acsabm.4c00318
- Bacakova L, Zikmundova M, Pajorova J, Broz A, Filova E, Blanquer A, et al. Nanofibrous scaffolds for skin tissue engineering and wound healing based on synthetic polymers. In: Applications of Nanobiotechnology. 2019. IntechOpen. Available from: http://dx.doi.org/10.5772/intechopen.88744
- Ye K, Kuang H, You Z, Morsi Y, Mo X. Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics. 2019;11:182. Available from: https://doi.org/10.3390/pharmaceutics11040182
- Eftekhari A, Maleki Dizaj S, Sharifi S, Salatin S, Rahbar Saadat Y, Zununi Vahed S, et al. The use of nanomaterials in tissue engineering for cartilage regeneration: current approaches and future perspectives. Int J Mol Sci. 2020;21(2):536. Available from: https://doi.org/10.3390/ijms21020536
- Hassan MI, Sultana N, Hamdan S. Bioactivity assessment of poly (ϵ-caprolactone)/hydroxyapatite electrospun fibers for bone tissue engineering application. J Nanomater. 2014;2014:Article ID 573238, 6 pages. Available from: http://dx.doi.org/10.1155/2014/573238
- Teber OO, Altinay AD, Mehrabani SAN, Tasdemir RS, Zeytuncu B, Genceli EA, et al. Polymeric hollow fiber membrane oxygenators as artificial lungs: a review. Biochem Eng J. 2022;180:108340. Available from: https://doi.org/10.1002/app.55121
- Bisceglie V. On antineoplastic immunity. Z Krebsforsch. 1934;40:122–40. Available from: https://doi.org/10.1007/BF01636399
- Oran DC, Gokulu I, Kizilel S. Nanoengineered biomaterials for pancreas regeneration. In: Nanoengineered Biomaterials for Regenerative Medicine. Micro and Nano Technologies. 2019. p. 443–57. Available from: http://dx.doi.org/10.1016/B978-0-12-813355-2.00019-3
- Membrane processes – medical applications. 2021.
- Xu G, Tan Y, Xu T, Yin D, Wang M, Shen M, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Biomater Sci. 2017;5(4):752–61.
- Kuang G, Lin X, Li J, Sun W, Zhang Q, Zhao Y. Electrospun nanofibers-derived functional scaffolds for cancer therapy. Chem Eng J. 2024;489:151253. Available from: https://doi.org/10.1016/j.cej.2024.151253
- Corral-Nájera K, Chauhan G, Sema-Saldívar SO, Martínez-Chapa SO, Aeinehvand MM. Polymeric and biological membranes for organ-on-a-chip devices. Microsyst Nanoeng. 2023;9:107. Available from: https://doi.org/10.1038/s41378-023-00579-z
- Yang X, Shi J, Shi B, Li J, Xue C, Ma J, et al. Micro- and nano-fibers for organ-on-a-chip: construction, applications, and prospects. Mater Today Bio. 2024;29:101322. Available from: https://doi.org/10.1016/j.mtbio.2024.101322
- Yang X, Li K, Zhang X, Liu C, Guo B, Wen W, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip. 2018;18(3):486–95. Available from: https://pubs.rsc.org/en/content/articlelanding/2018/lc/c7lc01224a
- Yu Y, Jin B, Chen J, Lou C, Guo J, Yang C, et al. Nerve-on-a-chip derived biomimicking microfibers for peripheral nerve regeneration. Adv Sci. 2023;10:Article 2207536. Available from: https://doi.org/10.1002/advs.202207536
- Tian L, Shi J, Li W, Zhang Y, Gao X. Hollow microfiber assembly-based endocrine pancreas-on-a-chip for sugar substitute evaluation. Adv Healthc Mater. 2024;13(21):e2302104. Available from: https://doi.org/10.1002/adhm.202302104
- Ma J, Li W, Tian L, Gao X. Preparation of tunable hollow composite microfibers assisted by microfluidic spinning and its application in the construction of in vitro neural models. Int J Bioprinting. 2024;10:264–78. Available from: https://doi.org/10.36922/ijb.1797
- Iqbal Z, Dilnawaz F. Formulation nanocarriers for vaginal drug delivery. Recent Pat Drug Deliv Formul. 2019;13(1):3–15. Available from: https://doi.org/10.2174/1872211313666190215141507
- Kharaziha M, Fathi MH, Edris H. Development of novel aligned nanofibrous composite membranes for guided bone regeneration. J Mech Behav Biomed Mater. 2013;24:9–20. Available from: https://doi.org/10.1016/j.jmbbm.2013.03.025
- Xu T, Ding Y, Liang Z, Sun H, Zheng F, Zhu Z, et al. Three-dimensional monolithic porous structures assembled from fragmented electrospun nanofiber mats/membranes: methods, properties, and applications. Prog Mater Sci. 2020;112:100656. Available from: https://doi.org/10.1016/j.pmatsci.2020.100656
- Wu S, Wang J, Jin L, Li Y, Wang Z. Effects of polyacrylonitrile/MoS₂ composite nanofibers on the growth behavior of bone marrow mesenchymal stem cells. ACS Appl Nano Mater. 2018;1(1):337–43. Available from: https://doi.org/10.1021/acsanm.7b00188
- Qian Y, Zhou X, Sun H, Yang J, Chen Y, Li C, et al. Biomimetic domain-active electrospun scaffolds facilitating bone regeneration synergistically with antibacterial efficacy for bone defects. ACS Appl Mater Interfaces. 2018;10(4):3248–3259. Available from: https://doi.org/10.1021/acsami.7b14524
- Chen CK, Liao MG, Wu YL, Fang ZY, Chen JA. Preparation of highly swelling/antibacterial cross-linked N maleoyl-functional chitosan/polyethylene oxide nanofiber meshes for controlled antibiotic release. Mol Pharm. 2020;17(9):3461–3476. Available from: https://doi.org/10.1021/acs.molpharmaceut.0c00504
- Rengifo AFC, Stefanes NM, Toigo J, Mendes C, Argenta DF, Dotto MER, et al. PEO-chitosan nanofibers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline for skin cancer treatment. Eur Polym J. 2019;119:335–343. Available from: https://doi.org/10.1016/j.msec.2019.110051
- Rezk AI, Unnithan AR, Park CH, Kim CS. Rational design of bone extracellular matrix mimicking tri-layered composite nanofibers for bone tissue regeneration. Chem Eng J. 2018;350:812–823. Available from: https://doi.org/10.1016/j.cej.2018.05.185
- Nalvuran H, Elin AE, Elin YM. Nanofibrous silk fibroin/reduced graphene oxide scaffolds for tissue engineering and cell culture applications. Int J Biol Macromol. 2018;114:77–84. Available from: https://doi.org/10.1016/j.ijbiomac.2018.03.072
- Bahrami S, Solouk A, Mirzadeh H, Seifalian AM. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos Part B Eng. 2019;168:421–431. Available from: https://doi.org/10.1016/j.compositesb.2019.03.044
- Garcia-Orue I, Gainza G, Garcia-Garcia P, Gutierrez FB, Aguirre JJ, Hernandez RM, et al. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing application. Int J Pharm. 2019;556:320–329. Available from: https://doi.org/10.1016/j.ijpharm.2018.12.010
- Cremar L, Gutierrez J, Martinez J, Materon L, Gilkerson R, Xu F, et al. Development of antimicrobial chitosan-based nanofiber dressings for wound healing applications. Nanomed J. 2018;5(1):6–14. Available from: https://nmj.mums.ac.ir/article_10049.html
- Jaganathan SK, Mani MP. Electrospun polyurethane nanofibrous composite impregnated with metallic copper for wound-healing application. 3 Biotech. 2018;8(8):327. Available from: https://doi.org/10.1007/s13205-018-1356-2
- Kouhi M, Jayarama Reddy V, Ramakrishna S. GPTMS-modified Bredigite/PHBV nanofibrous bone scaffolds with enhanced mechanical and biological properties. Appl Biochem Biotechnol. 2019;188(2):357–368. Available from: https://doi.org/10.1007/s12010-018-2922-0
- Rijal NP, Adhikari U, Khanal S, Pai D, Sankar J, Bhattarai N. Magnesium oxide-poly(ε-caprolactone)-chitosan-based composite nanofiber for tissue engineering applications. Mater Sci Eng B. 2018;228:18–27. Available from: https://www.researchgate.net/publication/322835927_Magnesium_oxide-polye-caprolactone-chitosan-based_composite_nanofiber_for_tissue_engineering_applications
- Lee D, Lee SJ, Moon JH, Kim JH, Heo DN, Bang JB, et al. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J Ind Eng Chem. 2018;66:196–202. Available from: https://khu.elsevierpure.com/en/publications/preparation-of-antibacterial-chitosan-membranes-containing-silver-2
- Khan MQ, Kharaghani D, Nishat N, Shahzad A, Hussain T, Khatri Z, et al. Preparation and characterizations of multifunctional PVA/ZnO nanofibers composite membranes for surgical gown application. J Mater Res Technol. 2019;8(1):1328–1334. Available from: http://dx.doi.org/10.1016/j.jmrt.2018.08.013
- Gu X, Li Y, Cao R, Liu S, Fu C, Feng S, et al. Novel electrospun poly(lactic acid)/poly(butylene carbonate)/graphene oxide nanofiber membranes for antibacterial applications. AIP Adv. 2019;9:65315. Available from: http://dx.doi.org/10.1063/1.5100109
- Waghmare VS, Wadke PR, Dyawanapelly S, Deshpande A, Jain R, Dandekar P. Starch based nanofibrous scaffolds for wound healing applications. Bioact Mater. 2017;3(3):255–266. Available from: https://doi.org/10.1016/j.bioactmat.2017.11.006
- Jatoi AW, Ogasawara H, Kim IS, Ni IQ. Polyvinyl alcohol nanofiber based three phase wound dressings for sustained wound healing applications. Mater Lett. 2019;241:168–171. Available from: http://dx.doi.org/10.1016/j.matlet.2019.01.084
- Ye H, Zhu J, Deng D, Jin S, Li J, Man Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J Biomater Sci Polym Ed. 2019;30(16):1505–1522. Available from: https://doi.org/10.1080/09205063.2019.1646628
- Toloue EB, Karbasi S, Salehi H, Rafienia M. Evaluation of mechanical properties and cell viability of poly(3-hydroxybutyrate)-chitosan/Al2O3 nanocomposite scaffold for cartilage tissue engineering. J Med Signals Sens. 2019;9(2):111–116. Available from: https://doi.org/10.4103/jmss.JMSS_56_18
- Rancan F, Contardi M, Jurisch J, Blume-Peytavi U, Vogt A, Bayer IS, et al. Evaluation of drug delivery and efficacy of ciprofloxacin-loaded povidone foils and nanofiber mats in a wound-infection model based on ex vivo human skin. Pharmaceutics. 2019;11(10):527. Available from: https://doi.org/10.3390/pharmaceutics11100527
- Kong X, He Y, Zhou H, Gao P, Xu L, Han Z, et al. Chondroitin sulfate/polycaprolactone/gelatin electrospun nanofibers with antithrombogenicity and enhanced endothelial cell affinity as a potential scaffold for blood vessel tissue engineering. Nanoscale Res Lett. 2021;16:62. Available from: https://doi.org/10.1186/s11671-021-03518-x
- Lai WY, Feng SW, Chan YH. in vivo investigation into effectiveness of Fe3O4/PLLA nanofibers for bone tissue engineering applications. Polymers. 2018;10(7):804. Available from: https://doi.org/10.3390/polym10070804