More Information
Submitted: June 05, 2025 | Approved: June 13, 2025 | Published: June 16, 2025
How to cite this article: Boujaoude J, Al-Bacha R, Abboud B. Chronic Pancreatitis with Stones: What is the Best Way to Treat? Arch Surg Clin Res. 2025; 9(1): 017-024. Available from:
https://dx.doi.org/10.29328/journal.ascr.1001086.
DOI: 10.29328/journal.ascr.1001086
Copyright license: © 2025 Boujaoude J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic pancreatitis; Stricture; Stone; Endotherapy; Surgery
Chronic Pancreatitis with Stones: What is the Best Way to Treat?
Joseph Boujaoude1, Rose Al Bacha1 and Bassam Abboud2*
1Department of Gastroenterology, Levant Hospital, Beirut, Lebanon
2Department of General Surgery, Geitaoui Hospital, Faculty of Medicine, Lebanese University, Beirut, Lebanon
*Address for Correspondence: Bassam Abboud, MD, Department of General Surgery, Geitaoui Hospital, Faculty of Medicine, Lebanese University, Beirut-Lebanon, Email: [email protected]
Pancreatic duct stones (PDS) are a common complication of chronic pancreatitis (CP). PDS can lead to duct obstruction and cause chronic abdominal pain. Ductal stone clearance, as well as short and long-term pain relief, is the cornerstone of endoscopic or surgical treatment. A step-up approach seems reasonable in pancreatic duct stone clearance. Extracorporeal shock wave lithotripsy (ESWL) combined with standard endoscopic retrograde cholangiopancreatography (ERCP) is as effective as a surgical approach for treating painful CP with less morbidity and medical costs. Therefore, endotherapy is considered a first-line therapy in selected patients. In case of insufficient pancreatic ductal clearance or strictures, advanced endoscopic techniques, per-oral pancreatoscopy (POP) with intraductal lithotripsy and/or endoscopic ultrasound-guided ductal drainage (EUS-PDD), will expand the role of the endoscopic approach. Because these new techniques are challenging, technically complex, and with high adverse events (AEs), they should be reserved for advanced tertiary care centers. Although there is increasing data that early surgical intervention may lead to better pain control and pancreatic duct stone clearance, surgery is reserved for patients failing endotherapy or patients with suspected malignancy.
Chronic pancreatitis (CP) is defined as a progressive inflammatory disease of the pancreas. CP undergoes fibrotic remodeling of the pancreatic tissue, which affects endocrine and exocrine functions. CP is increasing globally with an incidence of 1.6 to 23 per 100000 people [1]. During the natural course of CP, pancreatic duct stones (PDS) are observed in 50% of patients. In 32% of cases, calculi are combined with pancreatic duct stricture, and in 18% of cases are alone [2,3]. The dominant symptom of CP is pain. Its physiopathology is multifactorial. PD obstruction by stones and/or stricture. High intraductal pressure and ischemia from increased parenchymal pressure are the main pathophysiology mechanisms of pancreatic pain. Restoring pancreatic duct flow with complete pancreatic duct stone clearance and remodeling pancreatic duct stricture are the main objectives of the treatment of painful chronic pancreatitis. The aim of the present article is to review the appropriate current management of painful CP and to discuss the place of new techniques.
Diagnosis
To plan an appropriate treatment strategy for CP pain, it is crucial to document the number of PD stones (single, multiple), location (head, body, or tail), distribution (parenchymal or intraductal, main pancreatic duct or branches), and the nature (radiopaque or radiolucent). At the same time, we must evaluate any dominant PD structure to determine its length and location and to exclude malignancy [4-9]. Abdominal plain films and ultrasound are not sufficiently accurate to identify and locate PD stones. The best pre-interventional diagnostic tests should be CT, MRCP, and EUS [10-12]. These tests can better detect pancreatic calcifications (size, position, and nature) and visualize the pancreatic duct morphology (size, cartography, and dilation) and any anomalies (stricture and pancreas divisum).
Pancreatic duct stones
PD stones are generally composed of an inner nidus of small quantities of trace elements such as sulfur, nickel, chromium, iron, and chlorine. Successive outer shell layers of calcium carbonate and calcite are deposited and form the typical pancreatic stone. It is suggested that the reduction of pancreatic stone protein (PSP) results in calcium precipitation in the pancreatic juice and deposition in layers over the inner nidus [13,14]. This pathway of pancreatic lithiasis is like all etiologies of chronic pancreatitis, but very large intraductal pancreatic calculi characterize tropical pancreatitis.
In a recent study [15], 79.2% of calculi were radiopaque, 16% were radiolucent, and the rest were of the mixed type. Two-thirds of pancreatic calculi are single and found in the head and body, and in contrast, 15% of cases may be extensive and located in multiple areas.
Systems and techniques
Currently, endotherapy and surgery are the two available approaches to treat PDS and stricture in patients with painful CP. Non-steroidal anti-inflammatory drugs (NSAIDs) and opiates are the cornerstone of medication therapy following the WHO analgesic ladder. Other medications are considered to be beneficial for pain relief, like antioxidants, pregabalin, and S-ketamine. But their effects are still unclear, and larger-scale RCTs are still needed.
1. EndotherapyIn most cases, pancreatic stones are spiculated with high density and rigidity, making endoscopic treatment of pancreatic stones challenging. Two main procedures, ERCP and lithotripsy, can be used for stone removal and remodeling of pancreatic stricture.
a. Standard ERCP techniques: Inui reported the first pancreatic stone extraction by ERCP in 1983 [16], and by Cremer earlier. In 1985, Fuji and colleagues reported the first pancreatic stent placement [17].
- Stone retrieval: Balloons, baskets, or rat tooth forceps are used for stone retrieval from PD through the duodenum. Using balloons for PDS removal is safer than other systems and is recommended in clinical practice.
- Pancreatic stricture: A dominant PD stricture is defined as a significant narrowing of the main pancreatic duct with upstream dilation (> 6 mm) that prevents the flow of contrast [18]. Most PD strictures from chronic pancreatitis are fibrotic and refractory to balloon dilation alone. We recommend a combination of balloon dilation and a single plastic stent for the stricture for one year [19]. Stricture is classified as refractory if the dominant stricture persists or relapses after one year of using a single plastic stent. In this case, endoscopic options include multiple plastic stents side by side [20,21] or fully covered self-expandable metal stents (FCSEMS) [22].
ERCP fails in 3% - 10% of the cases with native anatomy. Many reasons make pancreatic duct access difficult or not feasible, like tight strictures or stones, anatomic duct variants that prevent deep cannulation, surgically altered foregut anatomy, luminal obstruction preventing access to the papilla, and a disconnected pancreatic duct. Currently, EUS has become a new frontier for accessing pancreatic ducts when ERCP fails, and it can offer an alternative to surgery. EUS-PDD can be performed in 2 ways: EUS-assisted pancreatic rendezvous (EUS-PRV) and EUS-guided pancreatico-gastrostomy (EUS-PG) [40].
- EUS-PRV: Bataille and colleagues reported it for the first time [41]. This approach is performed by puncturing the PD typically with a 19-gauge needle, which allows the passage of the guide-wire into the MPD. Guide-wire is advanced toward the papilla or surgical anastomosis across the papilla to the duodenum. Then the echoendoscope is exchanged for a duodenoscope, colonoscope, or enteroscope, which allows endoscopists to perform retrograde interventions: cannulation of PD, dilation of a stricture, extraction of stones, or placement of stents [41-44].
- EUS-PG: Francis and colleagues reported it for the first time in 2002 [45]. Generally, the procedure is achieved through the stomach. When the guidewire is placed into MPD as described above. Mechanical or cautery devices create a fistulous tract between the stomach and PD over the guidewire. Then, a plastic stent of 5F or 7F is placed transmurally and positioned toward the head of the pancreas. After 1 month, the tract becomes mature for further interventions: balloon dilation, stent replacement, or direct pancreatoscopy with direct visualization lithotripsy [46-48].
Pearce reported the first surgical stone removal in 1891 [49]. Surgery adopts one of three different approaches: resection of diseased tissue, ductal drainage, or a combination of the latter techniques. The nature of surgery depends on disease distribution and the size and morphology of the MPD [50]. Many surgical procedures were described. It includes the Whipple procedure, Puestow procedure, Frey procedure, total pancreatectomy, islet auto-transplantation, etc. The most commonly used surgical procedures are focused on duodenum-preserving resection of the head of the pancreas. In RCT, duodenum-preserving procedures have reduced in-hospital complications and better improvement of quality of life compared to duodenum-resecting procedures [51].
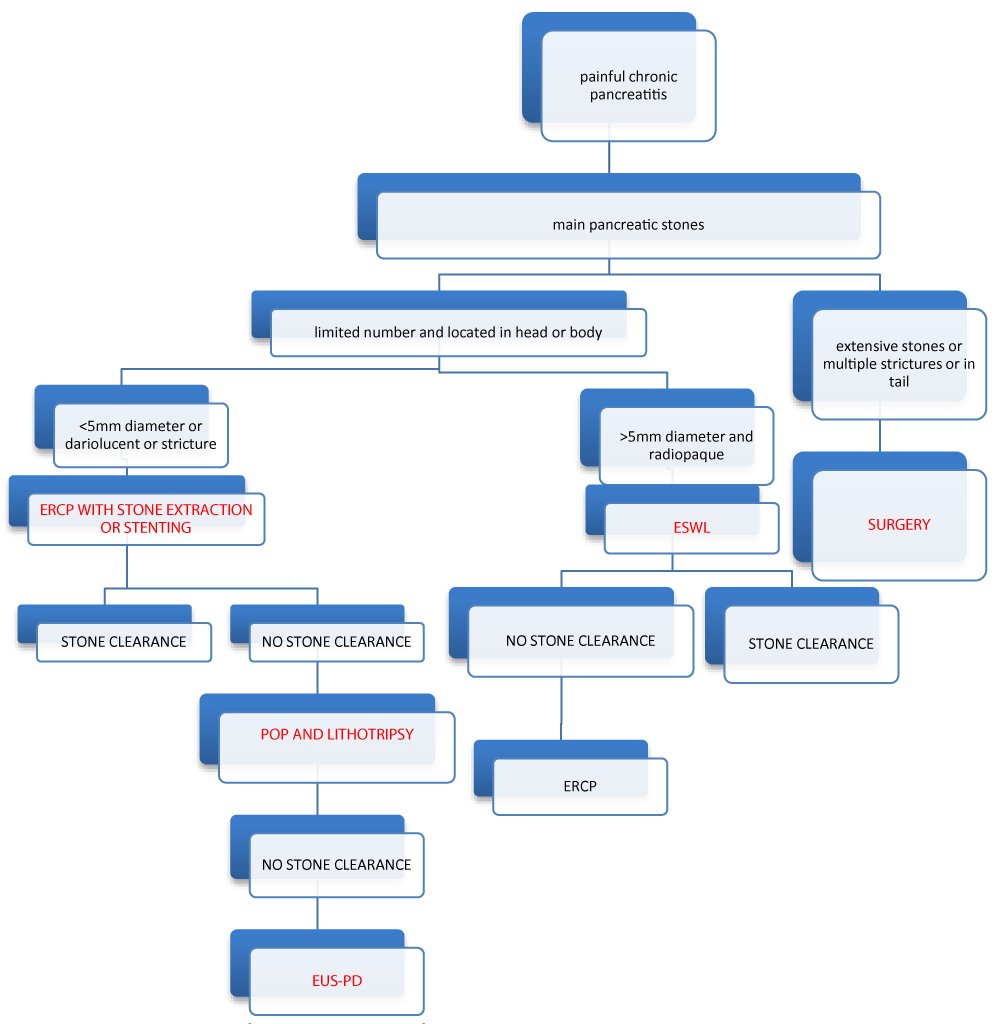
Algorithme
Indications
The current American Society for Gastrointestinal Endoscopy (ASGE) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines for treatment of PDS in CP are most often applied by endoscopist teams [52,53]. There is no indication of treatment in case of asymptomatic and uncomplicated CP.
Interventional therapy is indicated for patients with refractory pain after lifestyle modifications and pharmacotherapy. Endoscopic therapy and/or ESWL are now the first-line therapy for painful, uncomplicated CP with an obstructed MPD in the head and/or body of the pancreas. The selection of patients with the absence of MPD stricture, a short disease duration, absence of cigarette and alcohol intake, and complete removal of obstructive PDS had the best long-term outcome.
Guidelines recommend ERCP for MPD radiopaque stones smaller than 5mm and radiolucent stones located in the head and/or body of the pancreas. ESWL is recommended for the fragmentation of radiopaque obstructive MPD stones larger than 5mm, and in case of failure ERCP procedure. Isolated tail stones are not treated by ESWL because of the risk of spleen damage. ESWL is limited in its ability to address radiolucent stones, multiple strictures, and multiple stones in cases of ascites or coexistent pseudocyst. Coagulopathy must be corrected before ESWL. Guidelines suggest restricting the use of ERCP after ESWL in case of no spontaneous clearance of adequately fragmented pancreatic stones by ESWL. In ESWL, solitary stones, stones in the main pancreatic duct of the head, stones with a density on CT scans of < 820Hu, secretin or stenting before ESWL or ERCP delayed by 2 days are related to better outcome.
POP-guided intraductal lithotripsy (PGL) is an efficient alternative endotherapy for obstructing PDS. POP is especially indicated in the case of refractory and stent-dependent strictures with obstructing PDS, rendering the standard ERCP approach limited and inefficient. POP-PGL could directly visualize strictures for laser stricturoplasty and target calculi within the same session. So, it is suggested as third-line therapy when ERCP and ESWL fail to clear the MPD from stones, especially in the presence of pancreatic duct stricture.
Currently, EUS-PDD is an emerging technique. It is considered a salvage procedure after the technical failure of ERCP. EUS-RDV is selected if the papilla or anastomosis is easily accessible. EUS-PG is considered for patients with altered anatomy or in cases of guidewire failure to pass an obstruction during EUS-PRV. This technique is a challenging procedure with high rates of adverse events, and it is not widely adopted.
We know to date that surgery is widely accepted as the more effective treatment for painful CP. But endotherapy is considered the first-line treatment because it is a minimally invasive procedure.
All scientific societies favor ERCP and/or ESWL as a first-line approach to pancreatic duct drainage, given endoscopic advancements, minimal invasiveness, and low adverse events compared to surgery.
2 early monocenteric open-label randomized clinical trials (RCTs) suggest that surgery was superior to endoscopy to relieve pain in patients with obstructive CP [54,55]. These 2 trials suffer from several limitations. In the trial of Dite, et al. the results of the endoscopy and the surgery were not satisfactory (15% in endoscopy and 34% in surgery), and neither ESWL nor cumulative stenting was used. In the Cahen, et al. study, the number of patients included was very low (only 39 patients), so the results cannot be extrapolated to all painful obstructive CP patients. Also, data from the ESCAPE trial, a recent multicenter RCT, favor surgical intervention at an earlier stage to alleviate disease progression, leading to improved pain management [56]. But, this trial had two limitations, including the subjectivity of the pain score and the absence of shame-control. In a recent systematic review and meta-analysis, no difference between surgery and endotherapy was found in the short term. However, surgery was more efficient in pain relief than endoscopy in the long term [57].
Recent studies showed better clinical success with long-term results of ESWL combined with ERCP. The reason is due to better selection of patients for endotherapy and technical advancements. In fact, concerning clinical success, in a large prospective single-center series (1006 patients), ESWL achieved fragmentation of large PDS in 90% with less than 3 sessions, leading to pain relief in 84% of cases [58]. Likewise, in the most recent meta-analysis of 3668 patients, 86.3% of cases achieve complete fragmentation, leading to ductal clearance in 69.9% of cases and resulting in the absence of pain in over 50% [59]. Concerning long-term results, a recent systematic review showed that patients who remained asymptomatic at 2 years follow-up after complete pancreatic ductal clearance rarely experienced pain relapse thereafter [59]. Delhaye M, et al. followed for 14 years patients with painful CP treated by endotherapy. In their study, he reported long-term benefits for about two-thirds of these patients with a decrease in hospitalization rate and delayed impairment in exocrine pancreatic function [60]. Most of these patients were young, and maybe early intervention after the course of the disease. A Japanese RCT explored the efficacy of early endotherapy in 20 patients with mild painful CP in comparison with a wait-and-see policy. Preliminary results showed a benefit in terms of reducing the frequency of acute attacks and preventing gland atrophy [61].
One matter of debate is the benefit of the combination of ESWL and ERCP compared to ESWL alone. Only two studies, one randomized controlled trial and one retrospective clinical study, compared head-to-head ESWL alone to a combination with ERCP. No additional benefit in pain control was found in the systematic combination of the two procedures [62,63].
With the advancement of endoscopic techniques, new procedures have become an alternative to conventional ERCP and ESWL. When first introduced, several retrospective studies of POP-PGL showed high rates of stone clearance between 80% and 90% [64,65]. Likewise, two meta-analyses evaluated the performance of POP-PGL with either LL or EHL techniques. The first one includes 16 studies with a technical and clinical success rate of 76% and of 77% respectively [66]. The second one includes ten studies with large stones, with a mean size of 10.6mm, and found a technical success of 91% without a significant difference between EHL and LL [67]. Recently, a prospective multicenter RCT was published from Germany evaluating the efficacy of POP-PGL in painful CP. They selected patients with three or more stones, > 5 mm in diameter, and located in the pancreatic head or body. Complete stone clearance was achieved in 90% of cases [68]. The most recent systematic review and meta-analysis evaluating the safety and efficacy of POP-PGL treatment for symptomatic pancreatic duct stones included 17 studies, 5 prospective and 12 retrospective, with 441 patients [69]. The pooled complete stone clearance rate, clinical success rate, and adverse event rate were 81%, 90%, and 12%, respectively. POP-PGL can be considered a second-line endoscopic treatment for CP, evidenced by a high rate of safety and efficacy.
Finally, we review recent literature focused on EUS-PDD, encompassing clinical and technical success as well as complication rate. The overview showed a technical success rate ranging from 25 to 92%, long-term success within a range of 65% - 85%, and a complication rate spanning from 14 to 40% [70]. In 2018, EUS-PG, followed by antegrade pancreatoscopy via PG and intraductal lithotripsy, was described for the first time [71]. A recent systematic review compared head-to-head ERCP-guided to EUS-guided pancreatic access in altered anatomy. EUS-PD had the higher technical success rate. The success rates concerning pancreatic duct cannulation were 86% vs. 20%, pancreatography was 86% vs. 25%, and stent placement was 73% vs. 20% [72]. In one center, EUS-PD was also compared to enteroscopy-assisted ERCP on pancreatic duct access in altered anatomy. The technical success rates were 100% vs. 70, 7%, with a high rate of complications in the EUS approach. The overall clinical success rate, when the two approaches were combined, reached 85% [73].
In painful CP, pancreatic duct clearance is the mainstay of our treatment after medication treatment failure. Guidelines recommend a step-up approach. In selected cases, conventional ERCP and/or ESWL are offered as a first-line therapy. When endotherapy fails to sufficiently clear the pancreatic duct from stones or in altered anatomy, new endoscopic techniques may be offered as an alternative approach. POP is considered a second-line therapy. It can directly visualize the stones and the strictures and allow intraductal lithotripsy in the same session. EUS-PD is reserved for cases of ERCP technical failure or in patients with an inaccessible papilla. EUS-PD is a challenging and complex technique. It will be regarded as an alternative endoscopic approach in challenging and complex cases. Finally, surgery will be reserved for patients failing endotherapy or in cases with suspected malignancy. Despite all the technical and endoscopic advances, the management of painful chronic pancreatitis and pancreatic lithiasis remains insufficient and complex, with a high rate of complications. We need new devices that make direct access to the main pancreatic duct easier and powerful techniques capable of fragmenting calculi completely and quickly. As the pathophysiology of CP is multifactorial, new medications are needed as complementary treatment to endotherapy. What role does artificial intelligence play in the future?
Author contributions
Abboud B designed the research; Boujaoude J and Al Bacha R performed the research; Boujaoude J, Al Bacha R, and Abboud B analysed the data; Boujaoude J, Al Bacha R, and Abboud B wrote the paper.
- Gerges C, Beyna T, Neuhaus H. Management of pancreatic duct stones. Gastrointest Endosc Clin N Am. 2023;33(4):821-9. Available from: https://doi.org/10.1016/j.giec.2023.04.001
- Rosch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, et al. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34(10):765-71. Available from: https://doi.org/10.1055/s-2002-34256
- Dirweesh A, Trikudanathan G, Freeman ML. Endoscopic management of complications in chronic pancreatitis. Dig Dis Sci. 2022;67(5):1624-34. Available from: https://doi.org/10.1007/s10620-022-07391-1
- Toft J, Hadden WJ, Laurence JM, Lam V, Yuen L, Janssen A, et al. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: a systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol. 2017;92:17-23. Available from: https://doi.org/10.1016/j.ejrad.2017.04.009
- Niu X, Das S, Bhetuwal A, Bhetuwal A, Xiao Y, Sun F, Zeng L, et al. Value of diffusion-weighted imaging in distinguishing pancreatic carcinoma from mass-forming chronic pancreatitis: a meta-analysis. Chin Med J (Engl). 2014;127:3477-82. Available from: https://pubmed.ncbi.nlm.nih.gov/25269917/
- Fritscher-Ravens A, Brand L, Knofel WT, Bobrowski C, Topalidis T, Thonke F, et al. Comparison of endoscopic ultrasound-guided needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768-75. Available from: https://doi.org/10.1111/j.1572-0241.2002.07020.x
- Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-36. Available from: https://doi.org/10.1016/j.gie.2005.06.051
- Ohyama H, Mikata R, Ishihara T, Tsuyuguchi T, Sakai Y, Sugiyama H, et al. Efficacy of stone density on noncontrast computed tomography in predicting the outcome of extracorporeal shock wave for patients with pancreatic stones. Pancreas. 2015;44:422-8. Available from: https://doi.org/10.1097/mpa.0000000000000277
- Kolodziejczyk E, Jurkiewicz E, Pertkiewicz J, Wejnarska K, Dadalski M, Kierkus J, et al. MRCP versus ERCP in the evaluation of chronic pancreatitis in children: which is the better choice? Pancreas. 2016;45:1115-9. Available from: https://doi.org/10.1097/mpa.0000000000000644
- Midha S, Khajuria R, Shastri S, Kabra M, Garg PK. Idiopathic chronic pancreatitis in India: phenotypic characterization and strong genetic susceptibility due to SPINK1 and CFTR gene mutations. Gut. 2010;59:800-7. Available from: https://doi.org/10.1136/gut.2009.191239
- Luetmer PH, Stephens DH, Ward EM. Chronic pancreatitis: reassessment with current CT. Radiology. 1989;171:353-7. Available from: https://doi.org/10.1148/radiology.171.2.2704799
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc. 2002;55:507-11. Available from: https://doi.org/10.1067/mge.2002.122610
- Jing ZP. Ultrastructure and elemental composition of pancreatic stones. Zhonghua Wai Ke Za Zhi. 1990;28:421-3. Available from: https://pubmed.ncbi.nlm.nih.gov/2269050/
- Pitchumoni CS, Viswanathan KV, Varghese PJ, Banks PA. Ultrastructure and elemental composition of human pancreatic calculi. Pancreas. 1987;2(2):152-8. Available from: https://doi.org/10.1097/00006676-198703000-00005
- Tandan M, Nageshwar Reddy D, Talukdar R, Talukdar R, Vinod K, Kiran SVVS, et al. ESWL for large pancreatic calculi: report of over 5000 patients. Pancreatology. 2019;19(7):916-21. Available from: https://doi.org/10.1016/j.pan.2019.08.001
- Inui K, Nakae Y, Nakamura J. A case of non-calcified pancreatolithiasis which was removed by endoscopic sphincterotomy of the pancreatic duct. Gastroenterol Endosc. 1983;25(8):1246-53.
- Fuji T, Amano H, Harima K, Aibe T, Asagami F, Kinukawa K, et al. Pancreatic sphincterotomy and pancreatic endoprosthesis. Endoscopy. 1985;17(2):69-72. Available from: https://doi.org/10.1055/s-2007-1018460
- Delhaye M, Matos C, Deviere J. Endoscopic management of chronic pancreatitis. Gastrointest Endosc Clin N Am. 2003;13:717-42. Available from: https://doi.org/10.1016/s1052-5157(03)00070-9
- Jafri M, Javed S, Sachdev A, Lee D, Taur T, Goodman A, et al. Efficacy of endotherapy in the treatment of pain associated with chronic pancreatitis: a systematic review and meta-analysis. JOP. 2017;18:125-32. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5619873/
- Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-9. Available from: https://doi.org/10.1055/s-2005-921069
- Bove V, Tringali A, Valerii G. Endoscopic dilation of pancreatic duct strictures in chronic pancreatitis with multiple plastic stents: results of 48 patients. Gastrointest Endosc. 2017;85:AB236.
- Shen Y, Liu M, Chen M, Li Y, Lu Y, Zou X. Covered metal stent or multiple plastic stents for refractory pancreatic duct strictures in chronic pancreatitis: a systematic review. Pancreatology. 2014;14:87-90. Available from: https://doi.org/10.1016/j.pan.2013.12.005
- Sauerbruch T, Holl J, Sackmann M, Werner R, Wotzka R, Paumgartner G. Disintegration of a pancreatic duct stone with extracorporeal shock waves in a patient with chronic pancreatitis. Endoscopy. 1987;19(5):207-8. Available from: https://doi.org/10.1055/s-2007-1018284
- Choi KS, Kim MH. Extracorporeal shock wave lithotripsy for the treatment of pancreatic duct stones. J Hepatobiliary Pancreat Surg. 2006;13:86-93. Available from: https://doi.org/10.1007/s00534-005-1063-3
- Auge BK, Preminger GM. Update on shock wave lithotripsy technology. Curr Opin Urol. 2002;12:287-90. Available from: https://doi.org/10.1097/00042307-200207000-00005
- Li K, Lin T, Zhang C, Fan X, Xu K, Bi L, et al. Optimal frequency of shock wave lithotripsy in urolithiasis treatment: a systemic review and meta-analysis of randomized controlled trials. J Urol. 2013;190:1260-7. Available from: https://doi.org/10.1016/j.juro.2013.03.075
- Korpela T, Udd M, Tenca A, Lindström O, Halttunen J, Myrskysalo S, et al. Long-term results of combined ESWL and ERCP treatment of chronic pancreatitis. Scand J Gastroenterol. 2016;51:866-71. Available from: https://doi.org/10.3109/00365521.2016.1150502
- Tandan M, Reddy DN, Santosh D, Vinod K, Ramchandani M, Rajesh G, et al. Extracorporeal shock wave lithotripsy and endotherapy for pancreatic calculi—a large single center experience. Indian J Gastroenterol. 2010;29:143-8. Available from: https://doi.org/10.1007/s12664-010-0035-y
- Dumonceau JM, Costamagna G, Tringali A, Vahedi K, Delhaye M, Hittelet A, et al. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomized controlled trial. Gut. 2007;56:545-52. Available from: https://doi.org/10.1136/gut.2006.096883
- Karasawa Y, Kawa S, Aoki Y, Ochi Y, Unno H, Kiyosawa K, et al. Extracorporeal shock wave lithotripsy of pancreatic duct stones and patient factors related to stone disintegration. J Gastroenterol. 2002;37:369-75. Available from: https://doi.org/10.1007/s005350200051
- Li BR, Liao Z, Du TT, Ye B, Chen H, Ji JT, et al. Extracorporeal shock wave lithotripsy is a safe and effective treatment for pancreatic stones coexisting with pancreatic pseudocysts. Gastrointest Endosc. 2016;84:69-78. Available from: https://doi.org/10.1016/j.gie.2015.10.026
- Farnbacher MJ, Schoen C, Rabenstein T, Benninger J, Hahn EG, Schneider HT. Pancreatic duct stones in chronic pancreatitis: criteria for treatment intensity and success. Gastrointest Endosc. 2002;56:501-6. Available from: https://doi.org/10.1067/mge.2002.128162
- Tadenuma H, Ishihara T, Yamaguchi T, Tsuchiya S, Kobayashi A, Nakamura K, et al. Long-term results of extracorporeal shockwave lithotripsy and endoscopic therapy for pancreatic stones. Clin Gastroenterol Hepatol. 2005;3:1128-35. Available from: https://doi.org/10.1016/s1542-3565(05)00530-6
- Freeman ML. Mechanical lithotripsy of pancreatic duct stones. Gastrointest Endosc. 1996;44(3):333-6. Available from: https://doi.org/10.1016/s0016-5107(96)70175-x
- Nakajima M, Akasaka Y, Yamaguchi K, Fujimoto S, Kawai K. Direct endoscopic visualization of the bile and pancreatic duct systems by peroral cholangiopancreatoscopy (PCPS). Gastrointest Endosc. 1978;24(4):141-5. Available from: https://doi.org/10.1016/s0016-5107(78)73488-7
- Koch H, Stolte M, Walz V. Endoscopic lithotripsy in the common bile duct. Endoscopy. 1977;9(2):95-8. Available from: https://doi.org/10.1055/s-0028-1098497
- Howell DA. Endoscopic treatment of pancreatic duct stones using 10 F pancreatoscope and electrohydraulic lithotripsy. Gastrointest Endosc. 1999;50(6):829-33. Available from: https://doi.org/10.1016/s0016-5107(99)70168-9
- Lux G, Ell C, Hochberger D, Müller D, Demling L. The first successful endoscopic retrograde laser lithotripsy of common bile duct stones in man using a pulsed neodymium-YAG laser. Endoscopy. 1986;18(4):144-5. Available from: https://doi.org/10.1055/s-2007-1018356
- Vassar GJ, Chan KF, Teichman JM, Glickman RD, Weintraub ST, Pfefer TJ, et al. Holmium:YAG lithotripsy: photothermal mechanism. J Endourol. 1999;13(3):181-90. Available from: https://doi.org/10.1089/end.1999.13.181
- Trieu JA, Seven G, Baron TH. Endoscopic ultrasound guided pancreatic duct drainage. Gastrointest Endosc Clin N Am. 2024;34:501-10. Available from: https://doi.org/10.1016/j.giec.2024.02.002
- Bataille L, Deprez P. A new application of therapeutic EUS: main pancreatic duct drainage with a “pancreatic rendezvous technique”. Gastrointest Endosc. 2002;55(6):740-3. Available from: https://doi.org/10.1067/mge.2002.123621
- Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: report of 6 cases. Gastrointest Endosc. 2004;59(1):100-7. Available from: https://doi.org/10.1016/s0016-5107(03)02300-9
- Will U, Meyer F, Manger T, Wanzar I. Endoscopic ultrasound-assisted rendezvous maneuver to achieve pancreatic duct drainage in obstructive chronic pancreatitis. Endoscopy. 2005;37(2):171-3. Available from: https://doi.org/10.1055/s-2004-826151
- Motomura D, Irani S, Larsen M, Kozarek RA, Ross AS, Gan SI. Multicenter retrospective cohort of EUS-guided anterograde pancreatic duct access. Endosc Int Open. 2023;11(4):E358-65. Available from: https://doi.org/10.1055/a-2029-2520
- Francois E, Kahaleh M, Giovanini M, Matos C, Devière J. EUS-guided pancreatogastrostomy. Gastrointest Endosc. 2002;56(1):128-33. Available from: https://doi.org/10.1067/mge.2002.125547
- Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43(6):518-25. Available from: https://doi.org/10.1055/s-0030-1256333
- Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, et al. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75(1):56-64. Available from: https://doi.org/10.1016/j.gie.2011.08.032
- Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, et al. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14(4):377-82. Available from: https://link.springer.com/article/10.1007/s00534-006-1139-8
- Anderson DK, Frey CF. The evolution of the surgical treatment of chronic pancreatitis. Ann Surg. 2010;251(1):18-32. Available from: https://doi.org/10.1097/sla.0b013e3181ae3471
- Parekh D, Natarajan S. Surgical management of chronic pancreatitis. Indian J Surg. 2015;77(5):453-69. Available from: https://doi.org/10.1007/s12262-015-1362-0
- Guo S, Zhou Q, Yang J, Tao J, Zhang J, Wang H. Duodenum-preserving pancreatic head resection compared to pancreaticoduodenectomy: a systematic review and network meta-analysis of surgical outcomes. Front Surg. 2023;10:1107613. Available from: https://doi.org/10.3389/fsurg.2023.1107613
- Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51(2):179-93. Available from: https://doi.org/10.1055/a-0822-0832
- Gardner TB, Douglas A, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG clinical guideline: chronic pancreatitis. Am J Gastroenterol. 2020;115(3):322-39. Available from: https://doi.org/10.14309/ajg.0000000000000535
- Dite P, Ruzicka M, Zboril V. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy. 2003;35(7):553-8. Available from: https://doi.org/10.1055/s-2003-40237
- Cahen DL, Goumaa DJ, Laramee P, Nio Y, Rauws EA, Boermeester MA, et al. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology. 2011;141(5):1690-5. Available from: https://doi.org/10.1053/j.gastro.2011.07.049
- Issa Y, Kempeneers MA, Bruno MJ, Fockens P, Poley JW, Ahmed Ali U, et al. Effect of early surgery vs endoscopy-first approach on pain in patients with chronic pancreatitis: the ESCAPE randomized clinical trial. JAMA. 2020;323(3):237-47. Available from: https://doi.org/10.1001/jama.2019.20967
- Mendieta PJO, Sagae VMT, Ribeiro IB, de Moura DTH, Scatimburgo MVCV, Hirsch BS, et al. Pain relief in chronic pancreatitis: endoscopic or surgical treatment? A systematic review with meta-analysis. Surg Endosc. 2021;35(8):4085-94. Available from: https://doi.org/10.1007/s00464-021-08515-w
- Tandan M, Reddy DN, Santosh D, Vinod K, Ramchandani M, Rajesh G, et al. Extracorporeal shock wave lithotripsy and endotherapy for pancreatic calculi—a large single center experience. Indian J Gastroenterol. 2010;29(4):143-8. Available from: https://doi.org/10.1007/s12664-010-0035-y
- Van Huijgevoort NCM, Veld JV, Fockens P, Besselink MG, Boermeester MA, et al. Success of extracorporeal shock wave lithotripsy and ERCP in symptomatic pancreatic duct stones: a systematic review and meta-analysis. Endosc Int Open. 2020;8(8):E1070-85. Available from: https://doi.org/10.1055/a-1171-1322
- Delhaye M, Arvanitakis M, Verset G, Cremer M, Devière J. Long-term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol. 2004;2(12):1096-106. Available from: https://doi.org/10.1016/s1542-3565(04)00544-0
- Saito T, Nakai Y, Mizueo S, Isayama H, Sasahira N, Watanabe T, et al. A randomized-controlled trial of early endotherapy versus wait-and-see policy for mild symptomatic pancreatic stones in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2019;31(8):979-84. Available from: https://doi.org/10.1097/meg.0000000000001457
- Dumonceau JM, Costamagna G, Tringali A, Vahedi K, Delhaye M, Hittelet A, et al. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: randomized control trial. Gut. 2007;56:545-52. Available from: https://doi.org/10.1136/gut.2006.096883
- Vaysse T, Boytchev I, Antoni G, Croix DS, Choury AD, Laurent V, et al. Efficacy and safety of extracorporeal shock wave lithotripsy for chronic pancreatitis. Scand J Gastroenterol. 2016;51:1380-5. Available from: https://doi.org/10.1080/00365521.2016.1209688
- Attwel AR, Patel S, Kahaleh M, Raijman IL, Yen R, Shah RJ. ERCP with per-oral pancreatoscopy-guided laser lithotripsy for calcific chronic pancreatitis: a multicenter US experience. Gastrointest Endosc. 2015;82(2):311-8. Available from: https://doi.org/10.1016/j.gie.2015.01.020
- Attwell AR, Brauer BC, Chen YK, Yen RD, Fukami N, Shah RJ. Endoscopic retrograde cholangiopancreatography with per oral pancreatoscopy for calcific chronic pancreatitis using endoscope and catheter-based pancreatoscopes: a 10-year single-center experience. Pancreas. 2014;43(2):268-74. Available from: https://doi.org/10.1097/mpa.0b013e3182965d81
- Saghir SM, Mashiana HS, Mohan BP, Dhindsa BS, Dhaliwal A, Chandan S, et al. Efficacy of pancreatoscopy for pancreatic duct stones: a systematic review and meta-analysis. World J Gastroenterol. 2020;26(34):5207-19. Available from: https://doi.org/10.3748/wjg.v26.i34.5207
- McCarty TR, Sobani Z, Rustagi T. Per-oral pancreatoscopy with intraductal lithotripsy for difficult pancreatic duct stones: a systematic review and meta-analysis. Endosc Int Open. 2020;8(10):E1460-70. Available from: https://doi.org/10.1055/a-1236-3187
- Gerges C, Albers D, Schmitz L, Goni E, Cappello A, Schirra J, et al. Digital single-operator pancreatoscopy for the treatment of symptomatic pancreatic duct stones: a prospective multicenter cohort trial. Endoscopy. 2023;55:150-7. Available from: https://doi.org/10.1055/a-1870-3403
- Peiyao H, Hayat K, Wensong S, Yang J. Pancreatoscopy-guided lithotripsy for pancreatic duct stones: a systematic review and meta-analysis. Turk J Gastroenterol. 2024;Sept:1-13. Available from: https://doi.org/10.5152/tjg.2024.24110
- Will U, Fueldner F, Buechner T, Meyer F. Endoscopic ultrasonography-guided drainage of the pancreatic duct (EUS-PD) – indications and results with a literature review. J Clin Med. 2024;13:7709. Available from: https://doi.org/10.3390/jcm13247709
- James TW, Baron TH. Anterograde pancreatoscopy via EUS-guided pancreaticogastrostomy allows removal of obstructive pancreatic duct stones. Endosc Int Open. 2018;6:E735-8. Available from: https://doi.org/10.1055/a-0607-2484
- Basilyak K, Veldhuijzen G, Gerges C, Maubach J, Will U, Elmunzer BJ, et al. ERP-guided versus EUS-guided technique for pancreatography and cannulation in patients with a pancreaticojejunostomy stenosis: a systematic literature review. Endoscopy. 2020. Available from: https://doi.org/10.1055/a-1200-0199
- Kogure H, Sato T, Nakai Y. Performance of a new short-type double-balloon endoscope with advanced force transmission and adaptive bending for pancreaticobiliary intervention in patients with surgically altered anatomy: a propensity-matched analysis. Dig Endosc. 2019;31:86-93. Available from: https://doi.org/10.1111/den.13218